Solution
This is explained on the basis of ![]() , the equilibrium constant. In the given redox reaction, all reactants and products are solids at room temperature, so, there is no equilibrium between the reactants and products and hence the reactions does not occur at RT. At high temperature,
, the equilibrium constant. In the given redox reaction, all reactants and products are solids at room temperature, so, there is no equilibrium between the reactants and products and hence the reactions does not occur at RT. At high temperature, ![]() melts and values of
melts and values of ![]() increases. As a result, the value of
increases. As a result, the value of ![]() becomes more negative and hence the reaction proceeds rapidly.
becomes more negative and hence the reaction proceeds rapidly.
The reaction, 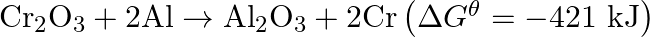 is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
The reaction, 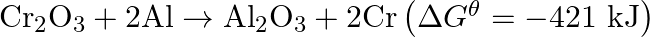 is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
