Ans: Given is the work function $0.1 \mathrm{eV}$, $$ \phi_{o}=h v_{0} $$ Class 12 Physics $\underline{\text { wuw. vedantu.com }}$ 1 $10 a-x=10 a-2.5 a=7.5 a$ from charge $9 q$
Calculate the threshold frequency of photon for photoelectric emission from a metal of work function
What is the Brewster angle for air to glass transition? (Refractive index of glass 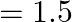 )
)
Ans: We are given the refractive index of glass to be, $\mu=1.5$ BreWster angle $=\theta$ Brewster angle is known to be related to refractive index as: $$ \begin{array}{l} \tan \theta=\mu \\...
At a certain location in Africa, a compass points 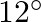 west of the geographic north. The north tip of the magnetic needle of a dip circle placed in the plane of magnetic meridian points above the horizontal. The horizontal component of the earth’s field is measured to be
west of the geographic north. The north tip of the magnetic needle of a dip circle placed in the plane of magnetic meridian points above the horizontal. The horizontal component of the earth’s field is measured to be 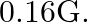 Specify the direction and magnitude of the earth’s field at the location.At a certain location in Africa, a compass points
Specify the direction and magnitude of the earth’s field at the location.At a certain location in Africa, a compass points 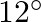 west of the geographic north. The north tip of the magnetic needle of a dip circle placed in the plane of magnetic meridian points above the horizontal. The horizontal component of the earth’s field is measured to be
west of the geographic north. The north tip of the magnetic needle of a dip circle placed in the plane of magnetic meridian points above the horizontal. The horizontal component of the earth’s field is measured to be 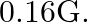 Specify the direction and magnitude of the earth’s field at the location.
Specify the direction and magnitude of the earth’s field at the location.
Ans: In the above question it is given that: Angle of declination, $\theta=12^{\circ}$ Angle of dip, $\delta=60^{\circ}$ Horizontal component of earth's magnetic field, $B_{H}=0.16 \mathrm{G}$...
Two long and parallel straight wires 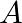 and
and 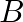 carrying currents of
carrying currents of 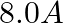 and
and 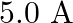 in the same direction are separated by a distance of
in the same direction are separated by a distance of 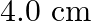 . Estimate the force on a
. Estimate the force on a 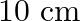 section of wire A.
section of wire A.
Ans: We are given the following: Current flowing in wire $A_{1} I_{A}=8.0 \mathrm{~A}$ Current flowing in wire $B, I_{B}=5.0 \mathrm{~A}$ Distance between the two wires, $r=4.0 \mathrm{~cm}=0.04...
A 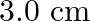 wire carrying a current of
wire carrying a current of 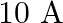 is placed inside a solenoid perpendicular to its axis. The magnetic field inside the solenoid is given to be
is placed inside a solenoid perpendicular to its axis. The magnetic field inside the solenoid is given to be 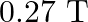 . What is the magnetic force on the wire?
. What is the magnetic force on the wire?
Ans: We are given the following: Length of the wire, $I=3 \mathrm{~cm}=0.03 \mathrm{~m}$ Current flowing in the wire, $1=10 \mathrm{~A}$. Magnetic field, $B=0.27 \mathrm{~T}$ Angle between the...
What is the magnitude of magnetic force per unit length on a wire carrying a current of 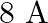 and making an angle of
and making an angle of 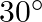 with the direction of a uniform magnetic field of
with the direction of a uniform magnetic field of 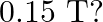
Ans: We are given the following: Current in the wire, $I=8 \mathrm{~A}$ Magnitude of the uniform magnetic field, $B=0.15 \mathrm{~T}$ Angle between the wire and magnetic field, $\theta=30^{\circ}$...
A long straight wire carries a current of 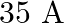 . What is the magnitude of the field
. What is the magnitude of the field 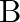 at a point
at a point 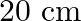 from the wire?
from the wire?
Ans: We are given the following: Current in the wire, $\mathrm{I}=35 \mathrm{~A}$ Distance of a point from the wire, $r=20 \mathrm{~cm}=0.2 \mathrm{~m}$ Magnitude of the magnetic field at this point...
State Biot-Savarts law. Derive an expression for magnetic field at the center of a circular coil of 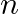 -turns carrying current
-turns carrying current 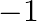 .
.
Ans: Biot - Savart law states that the magnetic field $\mathrm{dB}$ due to a current element dI at any point would be as following: $$ \begin{array}{l} \mathrm{dB} \propto \mathrm{dl} \\ \mathrm{dB}...
A straight wire carries a current of 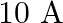 . An electron moving at
. An electron moving at 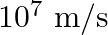 is at distance
is at distance 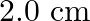 from the wire. Find the force acting on the electron if its velocity is directed towards the wire.
from the wire. Find the force acting on the electron if its velocity is directed towards the wire.
Ans: We are given the current through the straight wire to be, $I=10 \mathrm{~A}$ Speed of the electron, $\mathrm{v}=10^{7} \mathrm{~m} / \mathrm{s}$ Distance of electron from the wire,...
Give one difference each between diamagnetic and ferromagnetic substances. Give one example of each.
Ans: Diamagnetic substances are the ones that are weakly repelled by a magnet. For example, gold. Ferromagnetic materials are the ones that are strongly attracted by a magnet. For example, iron.
A steady current flows in the network What will be the magnetic field at the center of the network?
Ans: The magnetic field at the center of the network is zero. This is because, magnetic field at the center of the loop would just equal and opposite $i . e .$, magnetic field due PQR is equal and...
A cyclotron is not suitable to accelerate electron. Why?
Ans: A cyclotron is not suitable to accelerate electron as its mass is known to be less due to which they gain speed and step out of the dee immediately,
Two wires of equal lengths are bent in the form of two loops. One of the loops is square shaped whereas the other loop is circular. These are suspended in a uniform magnetic field and the same current is passed through them. Which loop will experience greater torque? Give reasons.
Ans: We know the expression for torque as, $$ \begin{array}{l} \tau=\mathrm{NIAB} \\ \Rightarrow \tau \propto \mathrm{A} \end{array} $$ Since, we know that the area of circular loops is more than...
What will be the path of a charged particle moving along the direction of a uniform magnetic field?
Ans: The path of a charged particle moving along the direction of a uniform magnetic field would be a straight line path as no force would act on the particle.
State two properties of the material of the wire used for suspension of the coil in a moving coil galvanometer.
Ans: Two properties of the material of the wire used for suspension of the coil in a moving coil galvanometer are: i. Non-Brittle conductor ii. Restoring Torque per unit twist should be small.
V.I graph for a metallic wire at two different temperatures Which of these two temperatures is higher and why?
Ans: From the graph: Slope $=\frac{\mathrm{i}}{\mathrm{V}}$ It is known that, $\frac{i}{V}=\frac{1}{R}$ It means that the smaller the slope, the larger the resistance.
Two heated wires of the same dimensions are first connected in series and then it’s parallel to a source of supply. What will be the ratio of heat produced in the two cases?
Ans: It is known that, $$ \begin{array}{l} H=I^{2} R t \quad\left(I=\frac{V}{R}\right) \\ \Rightarrow H=\frac{V^{2}}{R^{2}} \times R \times t \\ \Rightarrow H=\frac{V^{2}}{R} t \\ \Rightarrow H...
Resistivities of copper, silver and manganin are 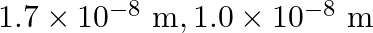 and
and 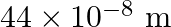 respectively which of these is the best conductor?
respectively which of these is the best conductor?
Ans: The resistance is directly proportional to specific resistance (resistivity), when length and area of cross-section is made constant. $R=\frac{\rho I}{A}$ Hence, silver is the best conductor as...
What is the work done in moving a 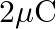 point change from corner
point change from corner 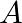 to corner
to corner 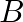 of a square
of a square 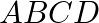 when a
when a 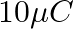 charge exists at the centre of the square?
charge exists at the centre of the square?
Ans: Point $A \& B$ are at the same distance from the point $O$. Hence,
Why does the electric field inside a dielectric decrease when it is placed in an external electric field?
Ans: The electric field that is present inside a dielectric decreases when it is placed in an external electric field because of polarisation as it creates an internal electric field which is...
State Gauss’s Theorem in electrostatics? Using this theorem define an expression for the field intensity due to an infinite plane sheet of change of charge density 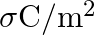 .
.
Ans: Gauss's Theorem has a statement that electric flux through a closed surface enclosing a charge $q$ in vacuum is $\frac{1}{\epsilon_{0}}$ times the magnitude of the charge enclosed $\mathrm{is}...
Prove that the energy stored in a parallel plate capacitor is given by 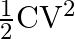
Ans: Let us suppose a capacitor is connected to a battery and it supplies a small amount of change dq at constant potential $V$, then a small amount of work done by the battery is given by $$...
Two dielectric slabs of dielectric constant 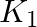 and
and 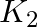 are filled in between the two plates, each of area A of the parallel plate capacitor as shown in the figure. Find the net capacitance of the capacitor? Area of each plate
are filled in between the two plates, each of area A of the parallel plate capacitor as shown in the figure. Find the net capacitance of the capacitor? Area of each plate 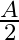
Ans: In the question, the two capacitors are in parallel Net Capacitance, $C=C_{1}+C_{2}$ $$ \begin{array}{l} \mathrm{C}_{1}=\frac{\mathrm{K}_{1}...
Describe schematically the equipotential surfaces corresponding to a) a constant electric field in the z-direction,
Ans: Equidistant planes which are parallel to the $x-y$ plane are the equipotential surfaces. b) a field that uniformly increases in magnitude but remains in a constant (say, z) direction, Ans:...
In a Van de Graaff type generator a spherical metal shell is to be a 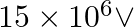 electrode. The dielectric strength of the gas surrounding the electrode is
electrode. The dielectric strength of the gas surrounding the electrode is 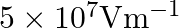 . What is the minimum radius of the spherical shell required? (You will learn from this exercise why one cannot build an electrostatic generator using a very small shell which requires a small charge to acquire a high potential.)
. What is the minimum radius of the spherical shell required? (You will learn from this exercise why one cannot build an electrostatic generator using a very small shell which requires a small charge to acquire a high potential.)
Ans: Given that, Potential difference is given as, $V=15 \times 10^{6} \mathrm{~V}$ Dielectric strength of the surrounding gas $=5 \times 10^{\top} \mathrm{Vm}^{-1}$ Electric field intensity is...
Two charges 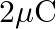 and
and 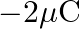 are placed at points
are placed at points  and
and 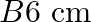 apart. a) Identify an equipotential surface of the system.
apart. a) Identify an equipotential surface of the system.
Ans: The situation is represented in the adjoining figure. An equipotential surface is the plane on which the total potential is zero everywhere. This plane is normal to line AB. The plane is...
The distance between the plates of a parallel plate capacitor is d. A metal plate of thickness 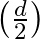 is placed between the plates. What will be the effect on the capacitance?
is placed between the plates. What will be the effect on the capacitance?
Ans: For air $C_{0}=\frac{A \in_{0}}{d}$ Thickness $t=\frac{d}{2}$ only when $k=\infty$ $$ \begin{array}{l} C_{0}=\frac{A \in_{0}}{d} \\ C_{\text {mat }}=\frac{A \in_{0}}{(d-t)} \end{array} $$ $$...
A stream of electrons travelling with speed 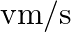 at right angles to a uniform electric field
at right angles to a uniform electric field 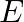 is deflected in a circular path of radius
is deflected in a circular path of radius 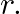 Prove that
Prove that 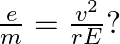
Ans: The path of the electron that is travelling with velocity $\mathrm{vm} / \mathrm{s}$ at right angle of $\bar{E}$ is of circular shape. It requires a centripetal in nature, $F=\frac{m v^{2}}{r}$...
If the amount of electric flux entering and leaving a closed surface are 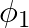 and
and 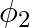 respectively. What is the electric charge inside the surface?
respectively. What is the electric charge inside the surface?
Ans: Net flux can be given as $=\phi_{2}-\phi_{1}$ Since $\phi=\frac{q}{\epsilon_{0}}$ $$ \Rightarrow \mathrm{Q}=\left(\phi_{2}-\phi_{1}\right) \in_{0} $$ The electric charge inside the surface can...
Draw one equipotential surfaces (1) Due to uniform electric field (2) For a point charge 
Ans: The diagrams are given as: Equipoterhibil Surfaces 3 $\mathrm{~ ( 2 c ) r o m}$
A parallel plate capacitor with air between the plates has a capacitance of 8pF 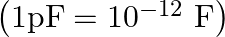 . What will be the capacitance if the distance between the plates is reduced by half and the space between them is filled with a substance of dielectric constant 6 ?
. What will be the capacitance if the distance between the plates is reduced by half and the space between them is filled with a substance of dielectric constant 6 ?
Ans: For air, capacitance can be expressed as, $C_{0}=\frac{A \in_{0}}{d}$ $$ C_{0}=8 \times p F=8 \times 10^{-12} F $$ Now $d^{\prime}=\frac{d}{2}$ and $K=6$ $$ \begin{array}{l} \Rightarrow...
Show mathematically that the potential at a point on the equatorial line of an electric dipole is Zero?
Ans: Electric potential at point on the equatorial line of an electric dipole can be expressed mathematically as: $$ \begin{array}{l} V=V_{P A}+V_{P B} \\ \Rightarrow...
What happens to the capacitance of a capacitor when a dielectric slab is placed between its plates?
Ans: The introduction of dielectric in a capacitor will reduce the effective charge on plate and therefore will increase the capacitance.
The plates of a charged capacitor are connected by a voltmeter. If the plates of the capacitor are moved further apart, what will be the effect on the reading of the voltmeter?
Ans: The relation between capacitance, area, distance and dielectric constant is $$ C=\frac{A E_{0}}{d} \Rightarrow C \propto \frac{1}{d} $$ Hence, if distance increases, capacitance decreases....
The distance of the field point on the equatorial plane of a small electric dipole is halved. By what factors will the electric field due to the dipole changes?
Ans: The formula $E x \frac{1}{r^{3}}$ gives the relation between electric field and distance. $$ \therefore E \propto \frac{1}{\left(\frac{r}{2}\right)^{3}} \Rightarrow E \propto \frac{8}{r^{3}} $$...
Force of attraction between two point electric charges placed at a distance d in a medium is 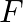 . What distance apart should these be kept in the same medium, so that force between them becomes
. What distance apart should these be kept in the same medium, so that force between them becomes 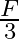 ?
?
Ans: If two point charges are $q_{1}$ and $q_{2}$ separated by distance $d$, it can be expressed as: $\mathrm{F}=\frac{\mathrm{Kq}_{1} \mathrm{q}_{2}}{\mathrm{~d}^{2}}$ Suppose if force becomes...
What is the work done in moving a  point change from corner
point change from corner 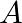 to corner
to corner 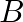 of a square
of a square 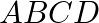 when a
when a 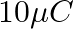 charge exists at the centre of the square?
charge exists at the centre of the square?
Ans: Point $A \& B$ are at the same distance from the point $O$. Hence,
Why does the electric field inside a dielectric decrease when it is placed in an external electric field?
Ans: The electric field that is present inside a dielectric decreases when it is placed in an external electric field because of polarisation as it creates an internal electric field which is...
Electric charge is uniformly distributed on the surface of a spherical balloon. Show how electric intensity and electric potential vary
a) on the surface Ans: Electric field intensity on the surface of the balloon would be, $$ E=\frac{\sigma}{\varepsilon_{0}} $$ Electric potential on the surface of the balloon would be, $$ V=\frac{K...
A sphere 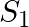 of radius
of radius 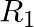 encloses a charge
encloses a charge 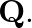 If there is another concentric sphere
If there is another concentric sphere 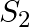 of radius
of radius 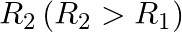 and there is no additional change between
and there is no additional change between 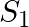 and
and  , then find the ratio of electric flux through
, then find the ratio of electric flux through 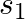 and
and  .
. 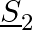
Ans: We may recall that the expression for electric flux through a surface enclosing charge q by Gauss's law is given by, $$ \phi=\frac{q}{E_{0}} $$ Where, $\varepsilon_{0}$ is the permittivity of...
A sphere 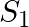 of radius
of radius 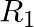 encloses a charge
encloses a charge 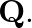 If there is another concentric sphere
If there is another concentric sphere 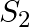 of radius
of radius 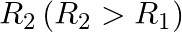 and there is no additional change between
and there is no additional change between 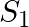 and
and  , then find the ratio of electric flux through
, then find the ratio of electric flux through 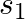 and
and  .
. 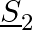
Ans: We may recall that the expression for electric flux through a surface enclosing charge q by Gauss's law is given by, $$ \phi=\frac{q}{E_{0}} $$ Where, $\varepsilon_{0}$ is the permittivity of...
Two charges 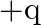 and
and 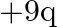 are separated by a distance of
are separated by a distance of 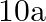 . Find the point on the line joining the two charges where electric field is zero.
. Find the point on the line joining the two charges where electric field is zero.
Ans: Let $p$ be the point (at $x$ distance from charge $+q$ ) on the line joining the given two charges where the electric field is zero. We know that the electric field at a point at r distance...
A particle of mass 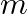 and charge a is released from rest in a uniform electric field of intensity E. Calculate the kinetic energy attained by this particle after moving a distance between the plates.
and charge a is released from rest in a uniform electric field of intensity E. Calculate the kinetic energy attained by this particle after moving a distance between the plates.
Ans: We have the electrostatic force on a charge a in electric field E given by. $\mathrm{F}=\mathrm{q} \mathrm{E} \quad \ldots \ldots(1)$ Also, we have Newton's second law of motion given by, $$...
If the net outward flux through the surface of the box were zero, could you conclude that there were no charges inside the box? Why or why not?
Ans: No. The net flux entering out through a body depends on the net charge contained in the body. If the net flux is given to be zero, then it can be inferred that the net charge inside the body is...
Careful measurement of the electric field at the surface of a black box indicate that the net outward flux through the surface of the box is 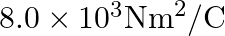 a) What is the net charge inside the box?
a) What is the net charge inside the box?
Ans: It is given that: Net outward flux through surface of the box, $\phi=8.0 \times 10^{3} \mathrm{Nr}^{2} / \mathrm{C}$. For a body containing net charge a, flux is given by...
What is the net flux of the uniform electric field of exercise 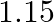 through a cube of side
through a cube of side 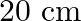 oriented so that its faces are parallel to the coordinate planes?
oriented so that its faces are parallel to the coordinate planes?
Ans: It is given that all the faces of the cube are parallel to the coordinate planes. Clearly, the number of field lines entering the cube is equal to the number of field lines entering out of the...
When a glass rod is rubbed with a silk cloth, charges appear on both. A similar phenomenon is observed with many other pairs of bodies. Explain how this observation is consistent with the law of conservation of charge. Ans: Rubbing is a phenomenon in which there is production of charges equal in
Ans: Since unlike charges attract and like charges repel each other, the particles 1 and 2 moving towards the positively charged plate are negatively charged whereas the particle 3 that moves...
Why can one ignore the quantization of electric charge when dealing with macroscopic i.e., large scale charge?
Ans: When dealing with macroscopic or large-scale charges, the charges used are huge in number as compared to the magnitude of electric charge. Hence, the quantization of electric charge is of no...
Explain the meaning of the statement ‘electric charge of a body is quantized”.
Ans: The statement 'electric charge of a body is quantized' suggests that only integral $(1,2,3,4, \ldots, \mathrm{n})$ number of electrons can be transferred from one body to another. This further...
An electric dipole when held at 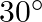 with respect to a uniform electric field of
with respect to a uniform electric field of 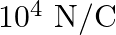 experiences a torque of
experiences a torque of 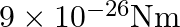 . Calculate dipole moment of the dipole?
. Calculate dipole moment of the dipole?
Ans: It is given that $$ \begin{array}{l} \theta=30^{\circ} \\ \tau=9 \times 10^{-26} \mathrm{Nm} \\ E=10^{4} \mathrm{~N} / \mathrm{C} \end{array} $$ Dipole moment P needs to be calculated. It is...
The graph shows the variation of voltage 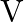 across the plates of two capacitors
across the plates of two capacitors 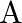 and
and 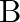 versus increase of charge
versus increase of charge 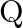 stored on them. Which of the two capacitors have higher capacitance? Give reason for your answer.
stored on them. Which of the two capacitors have higher capacitance? Give reason for your answer.
Ans: It is known that $c=\frac{Q}{V}$ Clearly, for a given charge $\mathrm{Q}$. $c \propto \frac{1}{V}$ Now, from the given graph, it is seen that $\mathrm{V}_{\mathrm{A}}
No two electric lines of force can intersect each other. Why?
Ans: Two electric lines of force can never intersect each other. Suppose if they intersect, then at the point of intersection, there can be two tangents drawn. These two tangents are supposed to...
A free proton and a free electron are placed in a uniform field. Which of the two experiences greater force and greater acceleration?
Ans: Force on both the electron as well as the proton in the uniform field would be equal because $\mathrm{F}=\mathrm{kq}$ and it is known that charge on both electron and proton are the same. On...
Define one coulomb.
Ans: Charge on a body is said to be 1 coulomb if it experiences a force of repulsion or attraction of $9 \times 10^{9} \mathrm{~N}$ from another equal charge when they are separated by a distance of...
Which physical quantity has its S.I. unit
1. 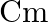 ?
?
2. 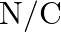 ?
?
Ans: 1.The S.I. unit of electric dipole moment is $\mathrm{Cm}$. 2. The S.I. unit of electric field intensity is N/C.
A charged rod P attracts a rod R whereas P repels another charged rod Q. What type of force is developed between  and
and  ?
?
Ans: Suppose that the rod $\mathrm{P}$ is negatively charged. As it attracts rod $\mathrm{R}$, it can be said that $\mathrm{R}$ is positively charged. Also, since $P$ repels rod $Q$, it can be said...
Does the force between two point charges change if the dielectric constant of the medium in which they are kept is increased?
Ans: Dielectric constant of a medium is given by $$ \begin{array}{l} k=\frac{F_{y}}{F_{M}}=\frac{\text { force between the charges in vaccum }}{\text { force between two charges in medium }} \\...
State the role of silica in the metallurgy of copper
Solution 8: The following processes are involved in the extraction of zinc from zinc blende: (i) Concentration: Zinc blende ore is crushed and the concentration done by froth- floatation process....
Write chemical reactions taking place in the extraction of zinc from zinc blende.
Solution 8: The following processes are involved in the extraction of zinc from zinc blende: (i) Concentration: Zinc blende ore is crushed and the concentration done by froth- floatation process....
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Solution 7: In the blast furnace reduction of iron oxides take place at different temperature ranges as shown below. At $500-800 \mathrm{~K}$ $$ \begin{array}{l} 3 \mathrm{Fe}_{2}...
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
Solution The common elements present in the anode mud are antimony, selenium, tellurium, silver, gold and platinum. These elements settle down under anode as anode mud because they are less reactive...
Out of 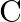 and
and 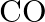 which is a better reducing agent at 673K?
which is a better reducing agent at 673K?
Solution 5: This can be explained thermodynamically, taking entropy and free energy changes into account (a) $C(s)+O_{2}(g) \rightarrow C O_{2}(g)$ (b) $2...
Explain:
(i) Zone refining
(ii) Column chromatography
Solution 4: (i) Zone refining: This method is used for production of semiconductors and other metals of very high purity, e.g., $\mathrm{Ge}, \mathrm{Si}, \mathrm{B}, \mathrm{Ca}$ and $\mathrm{In}$....
Explain:
(i) Zone refining
(ii) Column chromatography
Solution 4: (i) Zone refining: This method is used for production of semiconductors and other metals of very high purity, e.g., $\mathrm{Ge}, \mathrm{Si}, \mathrm{B}, \mathrm{Ca}$ and $\mathrm{In}$....
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Solution 3: $\Delta_{f} G^{o}$ of $\mathrm{Cu}_{2} \mathrm{~S}$ is more negative than $\Delta_{f} G^{\circ}$ of $\mathrm{CS}_{2} \mathrm{H}_{2} S$. So $C_{u} 2 S$ can not be reduced by carbon or...
What is the role of depressant in froth-floatation process?
Solution 2: The role of depressant is to prevent one type of sulphide ore particles from forming the froth with air bubbles. $\mathrm{NaCN}$ is used as a depressant to separate lead sulphide (PbS)...
Copper can be extracted by hydrometallurgy but not zinc. Explain.
Solution Copper can be extracted by hydrometallurgy but not zinc, this is because $E_{Z n^{2}+Z n}^{o}=-0.76 \mathrm{~V}$ lower than that of $E_{\mathrm{Cu}^{2+} / C i}^{o}=-0.34 \mathrm{~V}$ Hence,...
Is it true that under certain conditions, Mg can reduce 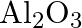 and
and 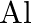 can reduce
can reduce 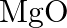 ? What are those conditions?
? What are those conditions?
Solution Yes, below $1350^{\circ} \mathrm{C}, \mathrm{Mg}$ can reduce $\mathrm{Al}_{2} \mathrm{O}_{3}$ and above $1350^{\circ} \mathrm{C}, \mathrm{Al}$ can reduce $\mathrm{MgO}$. This can be...
The reaction, 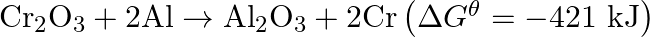 is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
Solution This is explained on the basis of $\mathrm{Keq}$, the equilibrium constant. In the given redox reaction, all reactants and products are solids at room temperature, so, there is no...
What is the significance of leaching in the extraction of aluminium?
Solution 2: Aluminium contains silica $\left(\mathrm{SiO}_{2}\right)$, iron oxide $\left(\mathrm{Fe}_{2} \mathrm{O}_{3}\right)$ and titanium oxide $\left(\mathrm{TiO}_{4}\right)$ as impurities....
Which of the ores mentioned can be concentrated by magnetic separation method?
Solution Ores which are magnetic in nature can be separated from non-magnetic gangue particles by magnetic separation method. For ex: ores of iron such as haemetite $\left(\mathrm{Fe} 2...
Specify the oxidation numbers of the metals in the following coordination entities:
(i) $\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)(\mathrm{CN})(\mathrm{en})_{2}\right]^{2+}$ Ans: Let us assume that the coordination number of Co is X. Therefore, we can write: $$...
What is meant by unidentate, bidentate and ambidentate ligands? Give two examples for each.
Ans: These are explained below: (i) Unidentate ligand Ligands with only one donor site are called unidentate ligands. For example, $\mathrm{Cl}$ and $\mathrm{NH}_{3}$ are unidentate ligands. (ii)...
Specify the oxidation numbers of the metals in the following coordination entities: (i) ![Rendered by QuickLaTeX.com \left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)(\mathrm{CN})(\mathrm{en})_{2}\right]^{2+}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-5830aca5c4a498a25851f5ca5b2a9534_l3.png)
Ans: Let us assume that the coordination number of Co is X. Therefore, we can write: $$ \begin{array}{l} x+0+(-1)+2(0)=+2 \\ x-1=+2 \\ x=+3 \end{array} $$ So, the coordination number of cobalt is...
What is meant by unidentate, bidentate and ambidentate ligands? Give two examples for each.
Ans: These are explained below: (i) Unidentate ligand Ligands with only one donor site are called unidentate ligands. For example, $\mathrm{Cl}$ and $\mathrm{NH}_{3}$ are unidentate ligands. (ii)...
Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Ans: All of them are explained below: (i) Coordination entity A central metal atom or anions connected to a set number of ions or molecules known as ligands comprises a coordination entity. For...
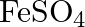 solution mixed with
solution mixed with 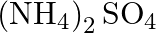 , solution in
, solution in 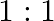 molar ratio gives the test of
molar ratio gives the test of 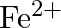 ion but
ion but 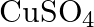 solution mixed with aqueous ammonia in
solution mixed with aqueous ammonia in 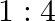 molar ratio does not give the test of
molar ratio does not give the test of 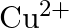 ion. Explain. why?
ion. Explain. why?
Ans: Let us see the reactions happening in both the cases. $$ \begin{array}{l} \left(\mathrm{NH}_{4}\right)_{2} \mathrm{SO}_{4}+\mathrm{FeSO}_{4}+6 \mathrm{H}_{2} \mathrm{O} \rightarrow...
Explain the bonding in coordination compounds in terms of Werner’s postulates.
Ans: Werner's theory is the first theory to explain the nature of bonding in coordination compounds. The main postulates of this theory are: (i) Two types of valencies, primary and secondary...
Calculate the overall complex dissociation equilibrium constant for the 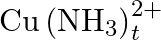 ion, given that
ion, given that 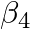 for this complex is
for this complex is 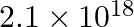 .
.
Ans: We are given the overall stability constant $\left(\beta_{s}\right)=2.1 \times 10^{13}$. The overall complex dissociation equilibrium constant is the reciprocal of the overall stability...
Write the formulas for the following coordination compounds: (i) Tetraamminediaquacobalt (III) chloride
Ans: The formula of Tetraamminediaquacobalt (III) chloride is $$ \left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)\left(\mathrm{NH}_{3}\right)_{4}\right] \mathrm{Cl}_{3} $$ (ii) Potassium...
A solution of glucose in water is labelled as 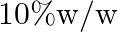 , that would be the molality and mole fraction of each component in the solution? If the density of solution is
, that would be the molality and mole fraction of each component in the solution? If the density of solution is 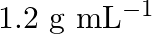 then what shall be the molarity of the
then what shall be the molarity of the
Solution 10 percent $w / w$ solution of glucose in water means $10 g$ glucose and $90 \mathrm{~g}$ of water. $10 \mathrm{~g}$ of glucose $=\frac{10}{180}=0.0555$ moles And $90 \mathrm{~g}$ of...
Density of solution 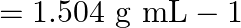 given Volume of
given Volume of
solution $-\frac{\text { mass }}{\text { density }}=\frac{100}{1.504}=66.5 \mathrm{~mL}$ Molarity of solution $$ \begin{array}{l} =\frac{\text { moles of sloution } \times 1000}{\text { Volume of...
Concentrated nitric acid used in laboratory work is 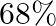 nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is
nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 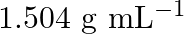 ?
?
Solution 4: $68 \%$ nitric acid by mass means that $68 \mathrm{~g}$ mass of nitric acid is dissolved in $100 \mathrm{~g}$ mass the solution. Molar mass of $\mathrm{HNO}_{3}=63 \mathrm{~g}...
Define the following terms:
(i) Mole fraction
(ii) Molality
(iii) Molarity
Solution 3: (i) Mole Fraction: It is defined as the ratio of the number of moles of the solute to the total number of moles in the solution. If $\mathrm{A}$ is the number of moles of solute...
Give an example of a solid solution in which the solute is a gas.
Solution Solution of hydrogen in palladium and dissolved gases in minerals
Calculate the osmotic pressure in pascals exerted by solution prepared by dissolving 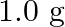 of polymer of molar mass 185,000 in
of polymer of molar mass 185,000 in 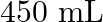 of water at
of water at 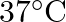 .
.
Solution $$ \begin{array}{l} \text { Given } \mathrm{V}=450 \mathrm{~mL}=0.45 \mathrm{~L} \\ =37^{\circ} \mathrm{C}=310 \mathrm{~K} \\ \mathrm{R}=8.314 \mathrm{kPaL} \mathrm{k}^{-1}...
Boiling point of water at 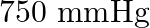 is
is 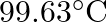 . How much sucrose is to be added to
. How much sucrose is to be added to 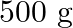 of water such that it boils at
of water such that it boils at 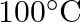 .
.
Solution Given $\Delta \mathrm{Tb}=100-99.63=3.37^{\circ}$ Mass of water w1 $=500 \mathrm{~g}$ Molar mass of water, $\mathrm{M}_{1}=18 \mathrm{~g} \mathrm{~mol}^{-1}$ Molar mass of sucrose,...
How can you determine the atomic mass of an unknown metal if you know its density and the dimension of its unit cell? Explain.
Ans: By knowing the density of an unknown metal and the dimension of its unit cell, the atomic mass of the metal can be determined. Let 'a' be the edge length of a unit cell of a crystal, 'd' be the...
What is meant by the term coordination number?
Ans: The number of nearest neighbors of any constituent particle present in the crystal lattice is called its coordination number.
How you distinguish between the following pairs of terms:
(i) Hexagonal close-packing and cubic close-packing? Ans: A 2-d hexagonal close-packing contains two types of triangular voids (a and b) as shown in figure 1. Let us call this 2-D structure as layer...
‘Stability of crystal is reflected in the magnitude of its melting point’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Ans: Higher the melting point, greater are the intermolecular forces of attraction between the atoms of a molecule and greater is the stability of that molecule. A substance with higher melting...
Niobium crystallises in body-centered cubic structure. If density is 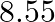
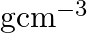 . Calculate the atomic radius of niobium using its atomic mass
. Calculate the atomic radius of niobium using its atomic mass 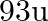 .
.
Ans: It is given that the density of niobium, $\mathrm{d}=8.55 \mathrm{gcm}^{-3}$ Atomic mass, $M=93 \mathrm{gmol}^{-1}$ As the lattice is bcc type, the number of atoms per unit cell, $z=2$ Let the...
A cubic solid is made of two elements  and
and 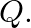 Atoms of
Atoms of 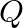 are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
Silver crystallises in the fcc lattice. If edge length of the cell is 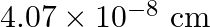 and density is
and density is 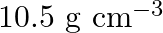 , calculate the atomic mass of silver.
, calculate the atomic mass of silver.
Ans: It is given that the edge length, $\mathrm{a}=4.07 \times 10^{-8} \mathrm{~cm}$ and density is $\mathrm{d}=$ $10.5 \mathrm{~g} \mathrm{~cm}^{3}$ As the lattice is foc type, the number of atoms...
Unit of electric potential is i) joule/coulomb ii) joule-coulomb iii) volt/metre iv) joule/coulomb-metre.
Correct option is (i)
Distinguish between electric potential and electric potential energy.
Electric potential at a place in an electric field is the amount of effort done to bring the unit positive charge from infinity to that point, whereas electric potential energy is the energy...
Stability of crystal is reflected in the magnitude of its melting point’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Ans: Higher the melting point, greater are the intermolecular forces of attraction between the atoms of a molecule and greater is the stability of that molecule. A substance with higher melting...
Niobium crystallises in body-centered cubic structure. If density is 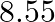
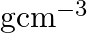 . Calculate the atomic radius of niobium using its atomic mass
. Calculate the atomic radius of niobium using its atomic mass 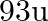 .
.
Ans: It is given that the density of niobium, $\mathrm{d}=8.55 \mathrm{gcm}^{-3}$ Atomic mass, $M=93 \mathrm{gmol}^{-1}$ As the lattice is bcc type, the number of atoms per unit cell, $z=2$ Let the...
A cubic solid is made of two elements  and
and  Atoms of
Atoms of  are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
Niobium crystallises in body-centered cubic structure. If density is 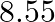
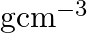 . Calculate the atomic radius of niobium using its atomic mass
. Calculate the atomic radius of niobium using its atomic mass 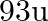 .
.
Ans: It is given that the density of niobium, $\mathrm{d}=8.55 \mathrm{gcm}^{-3}$ Atomic mass, $M=93 \mathrm{gmol}^{-1}$ As the lattice is bcc type, the number of atoms per unit cell, $z=2$
The ratio of electric force between two electrons to two protons separated by the same distance in the air is: A 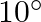 B
B 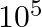 C
C 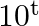 D None of these
D None of these
Correct option is (A) $10^{\circ}$ $\frac{\mathrm{F}_{\mathrm{e}}}{\mathrm{F}_{\mathrm{p}}}=10^{0}=1$, because force depends only on charges, distance and nature of medium and not type of charge. As...
A cubic solid is made of two elements  and
and  Atoms of
Atoms of  are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
A cubic solid is made of two elements  and
and  Atoms of
Atoms of  are at the corners of the cube and
are at the corners of the cube and  at the body-centre. What is the formula of the compound? What are the coordination numbers of
at the body-centre. What is the formula of the compound? What are the coordination numbers of  and
and 
Ans: It is given that the atoms of $\mathrm{Q}$ are present at the corners of the cube. Therefore, number of atoms of $Q$ in one unit cell $=8 \times(1 / 8)=1$ It is also given that the atoms of...
Two particles each of mass  and carrying charge
and carrying charge  are separated by some distance. If they are in equilibrium under mutual gravitational and electrostatic forces, then
are separated by some distance. If they are in equilibrium under mutual gravitational and electrostatic forces, then 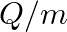 (in
(in 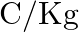 ) is of the order of:
) is of the order of:
A 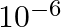
B 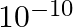
(C) 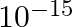
D 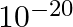
Correct option is (B) $10^{-10}$ Given : Gravitational force $=$ Electrostatics force Let the distance between the rwo charges be r. $$ \begin{array}{l} \Rightarrow \frac{\mathrm{G}(\mathrm{m})...
Electrostatic force and gravitational force differ in which respect?
In electrostatic force, the force of medium depends on charges while the force of the medium due to gravity does not depend on masses. There is no concept of induced mass.
Silver crystallises in the fcc lattice. If edge length of the cell is 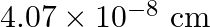 and density is
and density is 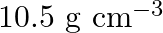 , calculate the atomic mass of silver.
, calculate the atomic mass of silver.
Ans: It is given that the edge length, $\mathrm{a}=4.07 \times 10^{-8} \mathrm{~cm}$ and density is $\mathrm{d}=$ $10.5 \mathrm{~g} \mathrm{~cm}^{3}$ As the lattice is foc type, the number of atoms...
Electrostatic force and gravitational force differ in which respect?
A Conservative force,
B Central force
c Principle of superposition
D Dependence on the intervening medium
Correct option is (D) Dependence on the intervening medium In electrostatic force, the force of the medium depends on charges while the force of the medium due to gravity does not depend on masses....
The excess (equal in number) number of electrons that must be placed on each of the two small spheres spaced 3cm apart with a force of repulsion between them to be 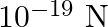 is :
is :
A 25
B 225
C 625
D 1250
Correct option is (C) 625 We need force of repulsion $=10^{-19} \mathrm{~N}$ Let $n$ no. of increase electrons be placed on each sphere $$ \begin{array}{l} \Rightarrow...
Copper crystallises into a fcc lattice with edge length 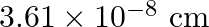 . Show that the calculated density is in agreement with its measured value of
. Show that the calculated density is in agreement with its measured value of 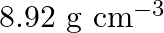
Ans: Edge length, $a=3.61 \times 10^{-6} \mathrm{~cm}$ As the lattice is fcc type, the number of atoms per unit cell, $z=4$ Atomic mass, $\mathrm{M}=63.5 \mathrm{~g} \mathrm{~mol}^{-1}$ We also know...
In comparison to the electrostatic force between two electrons, the electrostatic force between two protons is : A zero B smaller c same D greater
Correct option is(c) same Since the magnitude of the charge of electron and proton is the same, e, the electrostatic force between two protons is the same compared to the electrostatic force between...
What is a semiconductor? Describe the two main types of semiconductors and contrast their conduction mechanism.
Ans: Semiconductors are substances having conductance in the intermediate range $10^{-6}$ to $10^{4}$ ohm $^{-1} \mathrm{~m}^{-1}$. The two main types of semiconductors are: (i) n-type semiconductor...
Classify each of the following as being either a 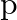 -type or an
-type or an 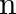 -type semiconductor.
-type semiconductor.
(i) Ge doped with In Ans: Ge (a group 14 element) is doped with In (a group 13 element). Therefore, a hole will be created and the semiconductor generated will be a p-type semiconductor. (ii) B...
Ferric oxide crystallises in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Ans: Let the number of oxide $\left(\mathrm{O}^{2-}\right)$ ions be $\mathrm{x}$. So, number of octahedral voids $=x$ It is given that two out of every three octahedral holes are occupied by ferric...
If 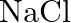 is doped with
is doped with 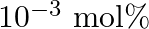 of
of 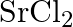 , what is the concentration of cation vacancies?
, what is the concentration of cation vacancies?
Ans: It is given that $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol} \%$ of $\mathrm{SrCl}_{2}$. This means that $100 \mathrm{~mol}$ of $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol}$ of...
In an A.C. circuit having resistance and capacitance :
A emf leads ahead of the current
B current lags behind the emf
c both the current and emf are in phase
D current leads ahead of the emf
Correct option is D current leads ahead of the emf $\mathrm{D}$ In circuits with primary capacitive loads, currently leads to the voltage. This is because the current must flow first to the two...
A parallel-plate air condenser of plate area A and separation d is charged to potential V and then the battery is removed. Now a slab of dielectric constant k is introduced between the plates. If Q, E, and W denote respectively the magnitude of the charge on each plate, the electric field between the plates (after the introduction of the dielectric slab) and work done on the system in the process of introducing the slab, then
A 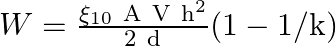
B 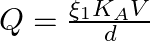
c 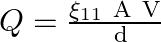
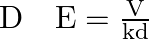
Correct option is A $\mathrm{W}=\frac{\varepsilon_{0} \mathrm{~A} \mathrm{~V} \mathrm{~h}^{2}}{2 \mathrm{~d}}(1-1 / \mathrm{k})$ C $Q=\frac{\varepsilon_{0} A V}{d}$ D $\quad E=\frac{V}{k d}$ As...
Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 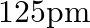 .
.
(i) What is the length of the side of the unit cell? Ans: For cubic close-packed structure: $$ \begin{array}{l} \mathrm{a}=2 \sqrt{2} \mathrm{r} \\ \Rightarrow 2 \sqrt{2}=125 \mathrm{pm} \\ =353.55...
A parallel plate capacitor with a dielectric slab of dielectric constant 3, filling the space between the plates, is charged to a potential V. The battery is then disconnected and the dielectric slab is withdrawn. It is then replaced by another dielectric slab of dielectric constant 2. If the energies stored in the capacitor before and after the dielectric slab is changed are 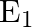 and
and 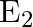 , then
, then 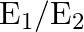 is:
is:
A 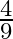
B 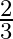
c 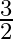
D 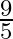
Correct option is B $\frac{2}{3}$ Let the charge stored by a capacitor with dielectric constant 3 be $Q$. Thus energy stored be $\frac{\mathrm{Q}^{2}}{2 \mathrm{C}_{1}}$ Since the charge remains the...
A parallel plate capacitor is first charged and then isolated, and a dielectric slab is introduced between the plates. The quantity that remains unchanged is:
A charge Q
B potential V
C capacity C
D energy U
The correct option is (A) charge Q When the capacitor is kept at a voltage, it gains charge. Now when the system is isolated, the charge present on the capacitor cannot change because of the law of...
If 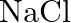 is doped with
is doped with 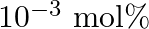 of
of 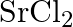 , what is the concentration of cation vacancies?
, what is the concentration of cation vacancies?
Ans: It is given that $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol} \%$ of $\mathrm{SrCl}_{2}$. This means that $100 \mathrm{~mol}$ of $\mathrm{NaCl}$ is doped with $10^{3} \mathrm{~mol}$ of...
When a dielectric slab is introduced between the two plates of the condenser then it’s capacity
A remains constant
B increases
C decreases
D may increase or decrease depending on the material of the dielectric slab
$\mathrm{~ O ~ W e i s c o d i s ~ T o p}$ Correct option is B increases As the dielectric slab is introduced there is some charge distribution in the slab and because of this, the electric field...
Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 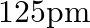 .
.
(i) What is the length of the side of the unit cell? Ans: For cubic close-packed structure: $$ \begin{array}{l} \mathrm{a}=2 \sqrt{2} \mathrm{r} \\ \Rightarrow 2 \sqrt{2}=125 \mathrm{pm} \\ =353.55...
Refractive index of a solid is observed to have the same value along all directions. Comment on the nature of this solid. Would it show cleavage property?
Ans: As isotropic solid has the same value of physical properties when measured along different directions. Therefore, the given solid, having the same value of refractive index along all...
In the LCR circuit if resistance increases, then the quality factor
A) increases finitely
B decreases finitely
C remains constant
D None of these
The correct option is B decreases finitely Quality factor $Q$ is given by: $$ \mathrm{Q}=\frac{\omega_{0} \mathrm{~L}}{\mathrm{R}} $$ Thus, as $\mathrm{R}$ increases Q definitely decreases.
The magnitude of force between two plates of a capacitor is 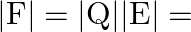 Transformer works on.
Transformer works on.
A 
B DC only
C Both  and DC
and DC
D AC more effectively than DC
The correct option is A AC only Transformer works on the principle of Electromagnetic induction i.e an emf is induced in the coil if the magnetic flux linked with the coil changes. Magnetic flux in...
Force between two plates of a capacitor is :
A 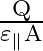
B 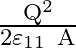
C 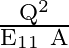
D none of these
Correct option is B $\frac{\mathrm{Q}^{2}}{2 \mathrm{\varepsilon}_{0} \mathrm{~A}}$ The magnitude of electric field by any one plate is $\mathrm{E}=\frac{\sigma}{2...
Why is glass considered a super cooled liquid?
Ans: Similar to liquids, glass has a tendency to flow, though very slowly. Therefore, glass is considered as a super cooled liquid. This is the reason that glass windows and doors are slightly...
Quality factor of series resonance circuit is given by
A 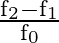
B 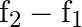
c) 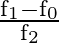
D 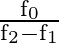
Correct option is D $\frac{\mathrm{f}_{0}}{\mathrm{f}_{2}-\mathrm{f}_{1}}$ Quality factor is a relation between stored energy and energy dissipation in a device or system. As per definition of...
What is Q-factor? Write its expression and write the conditions for its maximum value.
Q-factor: In LCR Circuit, the ratio of resonance frequency to the difference of its neighboring frequencies so that their corresponding current is $1 / \sqrt{2}$ times of the peak value, is called...
Why do solids have a definite volume?
Ans: The intermolecular forces of attraction that are present in solids are very strong, The constituent particles of solids have fixed positions i.e., they are rigid. Hence, solids have a definite...
Why are solids rigid?
Ans: The intermolecular forces of attraction that are present in solids are very strong. The constituent particles of solids cannot move from their positions i.e., they have fixed positions....
Using Biot-Savart’s law, obtain an expression for the magnetic field at a distance r meter from an infinitely long wire carrying a current of i ampere.
Biot savart law is the base of all magnetism and the effects of current. It states that the magnetic field intensity due to an element of a current-carrying wire at a point near the element is...
The principle on which transformer works is?
A Mutual induction
B Self induction
C Electromagnetic induction
D None of these
The correct option is A Mutual induction Mutual inductance is where the magnetic field generated by a coil of wire induces a voltage in an adjacent coil of wire. A transformer is a device...
$ Solid A is a very hard electrical insulator in solid as well as in molten state and melts at extremely high temperature. What type of solid is it?
Ans: The given properties are the properties of a covalent or network solid. Therefore, the given solid is a covalent or network solid. Examples of such solids include diamond ( $\mathrm{C}$ ) and...
A transformer is
A) A device for stepping up D.C.
B )A generator of current
C) Device for converting direct current into alternating current
D) A device for stepping up or down the voltage of  . supply
. supply
The correct option is D) A device for stepping up or down the voltage of $A C$. supply A transformer is a device that is used to either raise or lower voltages and currents in an electrical circuit....
Classify the following solids in different categories based on the nature of intermolecular forces operating in them: Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite, rubidium, argon, silicon carbide.
Ans: Potassium sulphate $\rightarrow$ ionic solid Tin Metallic $\rightarrow$ solid Benzene $\rightarrow$ Molecular (non-polar) solid Urea $\rightarrow$ Polar molecular solid Ammonia $\rightarrow$...
Explain the construction of the transformer.
For the construction of the transformer, you must have two coils having mutual inductance and a laminated steel core. The two coils are insulated from each other and from the steel core. The device...
Write the principle and explain the construction and working of a transformer. Define its efficiency.
Principle: The transformer is based on the principle of electromagnetic induction. The phenomenon of producing an induced emf due to the changes in the magnetic flux associated with a closed circuit...
Distinguish between
(i) Hexagonal and monoclinic unit cells Ans: Hexagonal unit cell: For a hexagonal unit cell $$ \begin{array}{l} a=b \neq c \\ \text { and } \alpha=\beta= \\ y=120^{\circ} \end{array} $$ Monoclinic...
Name the parameters that characterize a unit cell.
Ans: The six parameters that characterize a unit cell are as follows. (i) Its dimensions along the three edges, $a, b$, and $c$. These edges may or may not be equal. (ii) Angles between the edges....
Give the significance of a lattice point.
Ans: The significance of a lattice point is that each lattice point represents one constituent particle of a solid which may be an atom, a molecule (group of atom), or an ion.
A compound is formed by two elements  and
and 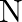 . The element
. The element 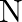 forms ccp and atoms of M occupy
forms ccp and atoms of M occupy 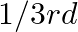 of tetrahedral voids. What is the formula of the compound?
of tetrahedral voids. What is the formula of the compound?
Ans: The ccp lattice is formed by the atoms of the element $\mathrm{N}$. Here, the number of tetrahedral voids generated is equal to twice the number of atoms of the element $\mathrm{N}$. According...
The frequency at which the impedance of the circuit boorens mavimum is
a 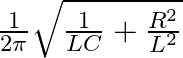
b 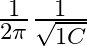
c 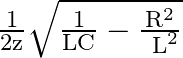
D 
Correct option is c $\frac{1}{2 \pi} \sqrt{\frac{1}{L C}-\frac{R^{2}}{L^{2}}}$ Megritude of admittanos $Y=\frac{1}{Z}$ is given by: $$ |\mathrm{Y}|=\frac{\mid \mathrm{H}^{2}...
A compound forms a hexagonal close-packed structure. What is the total number of voids in  of it? How many of these are tetrahedral voids?
of it? How many of these are tetrahedral voids?
A compound is formed by two elements $\mathrm{M}$ and $\mathrm{N}$. The element $\mathrm{N}$ forms ccp and atoms of M occupy $1 / 3 r d$ of tetrahedral voids. What is the formula of the compound?
The phase difference between the applied emf and the line current in an anti-resonant circuit at resonance is
A 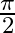 radian
radian
B 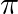 radian
radian
c 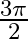 radian
radian
D zero
The correct option is D zero We know that phase difference is given by: $$ \phi=\arctan \left(\frac{\left(\mathrm{X}_{\mathrm{L}}-\mathrm{X}_{\mathrm{C}}\right)}{\mathrm{R}}\right) $$ at resonance...
The frequency at which the impedance of the circuit boorens mavimum is
a 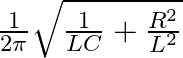
b 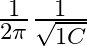
c 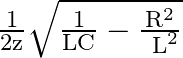
D 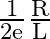
Correct option is c $\frac{1}{2 \pi} \sqrt{\frac{1}{L C}-\frac{R^{2}}{L^{2}}}$ Megritude of admittanos $Y=\frac{1}{Z}$ is given by: $$ |\mathrm{Y}|=\frac{\mid \mathrm{H}^{2}...
The resonent froquengy in an anti resonant circuitis:
a 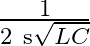
b 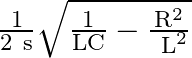
c 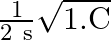
D 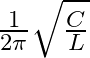
Correct option is a $\frac{1}{2 \pi \sqrt{L C}}$ For rescnance $$ \mathrm{X}_{\mathrm{L}}=\mathrm{X}_{\mathrm{E}} $$ $$ \begin{aligned} \mathrm{kL} &=\frac{1}{\mathrm{wC}} \\ \mathrm{w}...
What is the two dimensional coordination number of a molecule in a square close packed layer?
Ans: In a square close-packed layer, the molecule is in contact with four of its neighbours, Therefore, the two-dimensional coordination number of a molecule in a square close packed layer is $4 .$
The study of the earth’s magnetic field is called
A Terrestrial Magnetism
B Magneto Therapy
C Special Magnetism
D None of these
The correct option is A Terrestrial Magnetism
Power consumed in an AC circuit becomes zero if
A Inductance and resistance are both high.
B inductance and resistance are both low.
C inductance is very high and resistance is negligible.
D inductance is low and resistance is high.
The correct option is C inductance is very high and resistance is negligible. The power consumption in an AC circuit is zero when the circuit contains only inductance or capacitance since the...
Explain how much portion of an atom located at
(i) corner and
(ii) bodycentre of a cubic unit cell is part of its neighbouring unit cell.
Ans: (i) An atom located at the corner of a cubic unit cell is shared by eight adjacent unit cells. Therefore, 1 / 8 th portion of the atom is shared by one unit cell. (ii) An atom located at the...
What type of stoichiometric defect is shown by:
(i) $\mathrm{ZnS}$, Ans: ZnS shows Frenkel defect. (ii) $\mathrm{AgBr}$ Ans: AgBr shows Frenkel defect as well as Schottky defect.
The phase difference between the current and voltage at resonance is
A 0
B 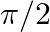
C 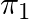
D 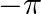
The correct option is (A) 0 Since reactive impedance at resonance is zero and we know that, $\tan \phi=\frac{X_{L}-X_{C}}{Z}$ but $\mathrm{X}_{\mathrm{L}}-\mathrm{X}_{\mathrm{C}}=0$ therefore...
What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
Ans: When a solid is heated, vacancy defects can arise. A solid crystal is said to have vacancy defects when some of the lattice sites are vacant. Vacancy defect leads to a decrease in the density...
An element with molar mass 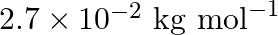 forms a cubic unit cell with an edge length
forms a cubic unit cell with an edge length 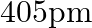 . If its density is
. If its density is 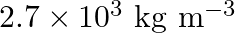 what is the nature of the cubic unit cell?
what is the nature of the cubic unit cell?
Ans: It is given that density of the element, $d=2.7 \times 10^{3} \mathrm{~kg} \mathrm{~m}^{-3}$ Molar mass, $M=2.7 \times 10^{-2} \mathrm{~kg} \mathrm{~mol}^{-1}$ Edge length, $\mathrm{a}=405...
What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your Solution.
Ans: Ferromagnetic substances would make better permanent magnets. In solid state, the metal ions of ferromagnetic substances are grouped together into small regions. These regions are called...
A group 14 element is to be converted into n-type semiconductor by doping it with a suitable impurity. To which group should this impurity belong?
Ans: An n-type semiconductor conducts because of the presence of extra electrons. Therefore a group 14 element can be converted to n-type semiconductor by doping it with a group 15 element.
Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example.
Ans: The colour develops because of the presence of electrons in the anionic sites. These electrons absorb energy from the visible part of radiation and get excited. For example, when crystals of...
Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
Ans: When a cation of higher valence is added to an ionic solid as an impurity to it, the cation of higher valence replaces more than one cation of lower valence so as to keep the crystal...
Complete the following acid-base reactions and name the products:
i. $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{2}+\mathrm{HCl} \rightarrow$ Ans: n-propylammonium chloride ii. $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3}...
Calculate the vapour pressure of water for this solution and its relative lowering.
Solution 9: \begin{tabular}{l} \hline $\mathrm{P} \square=23.8 \mathrm{~mm} \mathrm{Hg}$ \\ $\mathrm{W} 2=50 \mathrm{~g}, \mathrm{M}_{2}($ urea $)=60 \mathrm{~g} \mathrm{~mol}^{-1}$ \\ $\mathrm{w}...
Heny’s law constant for 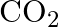 in water is
in water is 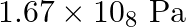 at
at 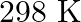 . Calculate the quantity of
. Calculate the quantity of 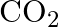 in
in 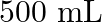 of soda water when packed under
of soda water when packed under 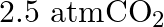 pressure at
pressure at 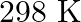 .
.
Solution 7: $$ \mathrm{KH}=1.67 \times 10_{8} \mathrm{~Pa} $$ $$ \begin{array}{l} \hline P_{A}^{o}=450 \mathrm{~mm}, P_{B}^{o}=700 \mathrm{~mm}, P_{\text {total }}=600 \mathrm{~mm} \\ \text { As...
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes: (i) ![Rendered by QuickLaTeX.com \mathrm{K}_{5}\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-32cbfc6fdf62516d53f060567ba2033d_l3.png)
Ans: The complex will be $\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{8}\right]^{3}$. Oxidation state of cobalt will be $=x-6=-3$ The oxidation state is $=+3$ Coordination number...
Discuss the nature of bonding in metal carbonyls.
Ans: The carbon metal linkages in metal carbonyls are characterized by both s and p. M-Ca bond consists of a donation into an empty metal orbital of a lone pair of electrons on the carbonyl carbon....
What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Ans: In $\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3 v}$ the oxidation state of $\mathrm{Cr}$ is in $+3$ oxidation state, which will cause the configuration of $\mathrm{Cr}$ as...
Using IUPAC norms Write the systematic names of the following:
(i) $\quad\left[\mathrm{CO}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}$ Ans: The IUPAC name of the compound is Hexaamminecobalt(III) chloride. (ii)...
Calculate the mass of urea (NH2CONH2) required in making 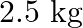 of
of 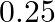 molal aqueous solution.
molal aqueous solution.
Solution 4: $0.25$ molal aqueous solution to urea means that Moles of urea $=0.25$ mole Mass of solvent $(\mathrm{NH} 2 \mathrm{CONH} 2)=60 \mathrm{~g} \mathrm{~mol}^{-1}$ $\therefore 0.25$ mole of...
Calculate the molarity of each of the following solution: (a) 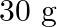 of
of 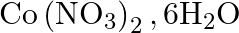 in
in 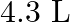 of
of
solution (b) $30 \mathrm{~mL}$ of $0.5 \mathrm{M} \mathrm{H}_{2} \mathrm{SO}_{4}$ diluted to $500 \mathrm{~mL}$. Solution 3: (a) Molar mass of $\mathrm{Co}\left(\mathrm{NO}_{3}\right)_{2}, 6...
Calculate the mole fraction of benzene in solution containing 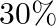 by mass in carbon tetrachloride.
by mass in carbon tetrachloride.
Solution 2: $30 \%$ by mass of $\mathrm{C} 6 \mathrm{H} 6$ in $\mathrm{CCl} 4 \Rightarrow 30 \mathrm{~g} \mathrm{C} 6 \mathrm{H} 6$ in $100 \mathrm{~g}$ solution $\therefore$ Number of moles of...
Calculate the mass percentage of benzene 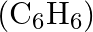 and carbon tetrachloride
and carbon tetrachloride 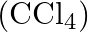 if
if 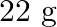 of benzene is dissolved in
of benzene is dissolved in 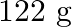 of carbon tetrachloride.
of carbon tetrachloride.
Solution Mass percentage of $$ \begin{array}{l} C_{6} H_{6}=\frac{\text { Mass of } \mathrm{C}_{6} \mathrm{H}_{6}}{\text { Total mass of the solution }} \times 100 \% \\ =\frac{\text { Mass of }...
Classify each of the following solids as ionic, metallic, molecular, network (covalent) or amorphous.
(i) Tetra phosphorus decoxide $\left(\mathrm{P}_{4} \mathrm{O}_{20}\right)$ (ii)Ammonium phosphate $\left(\mathrm{NH}_{4}\right)_{3} \mathrm{PO}_{4}$ (iii) $\mathrm{SiC}$ (iv) $\mathrm{I}_{2}$ (v)...
What makes a glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
Ans: The arrangement of the constituent particles makes glass different from quartz. In glass, the constituent particles have short range order, but in quartz, the constituent particles have both...
Define the term ‘amorphous’ give a few examples of amorphous solids.
Ans: Amorphous solids are the solids that have their constituent particles of irregular shape and have short range order. These solids are isotropic in nature and melt over a range of temperature....
How are synthetic defergents better than soup?
Solution Soaps work in soft water. However, they are not effective in hard water. In contrast, synthetic detergents work both in soft water and hard water. Therefore, synthetic detergents are better...
What problem arises in using alitame as artificial sweetener?
Solution Alitame is a high potency sweetener. It is difficult to control the sweetness of food while using alitame as an artificial sweetener.
Name a sweetening agent used in the preparation of sweets for a diabetic patient
Solution Artificial sweetening agents such as saceharin, alitame, and aspartame can be used in preparing sweets for diabetie paticats. Question 19: What problem arises in using alitame as artificial...
What are artificial sweetening agents? Give two examples.
Solution Artificial sweetening agents are chemicals that sweeten food. However, unlike natural sweeteners, they do not add calories to our body. They do not harm the human body. Some artificial...
Why is use of aspartame limited to cold foods and drinks?
Solution Aspartame becomes unstable at cooking temperature. This is the reason why its use is limited to cold foods and drinks.
What are food preservatives?
Solution Food preservatives are chemicals that prevent food from spoilage due to microbial growth. Table salt, sugar, vegetable oil, sodium benzoate (ChHsCOONa), and salts of propanoic acid are...
What is tincture of iodine? What is its use?
Solution Tincture of iodine is a $2-3$ percent solution of wodine in alcohol - water mixture, It is applied to wounds as an antiseptic.
What are the main constituents of dettol?
Solution The main constituents of dettol are chloroxylenol and a-terpineol. CC1=CC(O)=CC(C)C1Cl CC1=CCC(C(C)(C)Br)CC1 Chatisiars a-ticyanat
Name a substance which can be used as an antiseptic as well as disinfectant.
Solution Phenol can be used as an antiseptic as well as a disinfectant. $0.2$ peroent solution of phenol is used as an antiseptic, while 1 per cent of its solution is used as a disinfectant.
Why are cimetidine and ranitidine better antacids than sodium hydrogen carbonate or magnesium or aluminium hydroxide?
Solution Cimetidine and rantidine are better antacids as they control the root eause of acidity. These drugs prevent the interaction of histamine with the receptors present in the stomach walls....
How do antiseptics differ from disinfectants? Give one example of each.
Solution Antiseptics and disinfectants are effective against micro-organisms. However, antiseptics are applied to the living tisswes such as wounds, cuts, ulcers, and diseased skin surfaces, while...
What is meant by the term ‘broad spectrum antibiotics’? Explain.
Solution 9: Antibiotics that are effective against a wide range of gram-positive and gram-negative bacteria are known as broad spectrum antibiotics. Chloramphenicol is a broud spectrum antibiotic....
Low level of noradrenaline is the cause of depression. What types of drugs are needed to cure this problem? Name two drugs
Solution \&: Anti-depressant drugs are needed to counteract the effect of depression. These drugs inhibit Enzymes catalysing the degradation of the neurotransmitter, noradrenaline. As a result, the...
While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other?
Solution Specific drugs affect particular receptors. Antacids and anti-allergie drugs work on different receptors. This is the reason why antacids and anti-allergic drugs do not interfere with each...
Which foroes are involved in holding the drugs to the active site of enzymes?
Solution Either of the following forces can be involved in holding drugs to the active sites of enzymes, (i) Ionic bonding (ii) Hydrogen bonding (iii) Dipole - dipole interaction (iv) van der Waals...
Define the tern chemotherapy.
Solution The use of chemicals for therapeutic effect is called chemotherapy. For example: the use of chemicals in the diagnosis, prevention, and treatment of diseases
Name the macromolecules that are chosen as drug targets.
Solution The macromolecules chosen as drug targets are carbohydrates, lipids, proteins, and nucleic acids,
Explain the term target molecules or drug targets as used in medicinal chemistry.
Solution In medicinal chemistry, drug targets refer to the key molecules involved in certain metabolic pathways that result in specific diseases, Carbohydrates, proteins, lipids, and nucleic acids...
Why do we need to classify drugs in different ways?
Solution The classification of drugs and the reasons for classification are as follows: (i) On the basis of pharmacological effect: This classification provides doctors the whole range of drugs...
Complete the following acid-base reactions and name the products:
i. $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{NH}_{2}+\mathrm{HCl} \rightarrow$ Ans: n-propylammonium chloride ii. $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3}...
Arrange the following in increasing order of their basic strength: i. 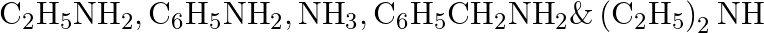
Ans: Considering the inductive effect of alkyl groups $\mathrm{NH}_{3}, \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$ and $\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$ can be...
An organic compound with the molecular formula 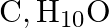 forms 2,4 -DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2 -benzenedicarboxylic acid. Identify the compound.
forms 2,4 -DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2 -benzenedicarboxylic acid. Identify the compound.
Ans: It is because the chemical $\left(\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}\right)$ generates a derivative of 2,4 -dnp and reduces the reagent of Tollen. The chemical must thus be an aldehyde....
Draw structures of the following derivatives.
$\mathrm{H}_{3} \mathrm{C} \longrightarrow{\mathrm{C}}_{2}^{\mathrm{H}_{2}} \mathrm{CHO}+\mathrm{H}_{3} \mathrm{C} \longrightarrow \stackrel{\mathrm{H}_{2}}{\mathrm{C}}-\mathrm{CHO} \longrightarrow...
Write the IUPAC names of the following ketones and aldehydes. Whenever possible, give also common names.
(i) $\mathrm{CH}_{3} \mathrm{CO}\left(\mathrm{CH}_{2}\right)_{4} \mathrm{CH}_{3}$ Ans: The IUPAC name of the given compound is Heptan-2-one and its common name is Methyl n-pentyl ketone. (ii)...
Draw the structures of the following compounds:
(i) 3-Methylbutanal Ans: The structure of 3-Methylbutanal is given below: CC(C)CC=O (ii) p-Nitropropiophenone Ans: The structure of p-Nitropropiophenone is given below: (viii) Hex-2-en-4-ynoic acid...
Name the following compounds according to IUPAC system of nomenclature: (i) 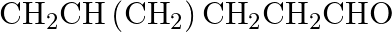
Ans: The IUPAC name of the given compound is 4-Methylpentanal, (ii) $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COCH}\left(\mathrm{C}_{2} \mathrm{H}_{5}\right) \mathrm{CH}_{2} \mathrm{CH}_{2}...
Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Ideatify the functional group (s) present in the molecule. 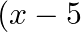 to 109
to 109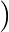
Solution 5: Functional groups present in the molecule are: Hy trophobic part (i) Ether, and (ii) primary alcoholic group wime yodansu cion 10 "odididu Lice onun: r.toning $\mathrm{R}-\mathrm{C}=$...
