Solution
Yes, below ![]() can reduce
can reduce ![]() and above
and above ![]() can reduce
can reduce ![]() . This can be inferred from
. This can be inferred from ![]() vs T plots.
vs T plots.
Is it true that under certain conditions, Mg can reduce 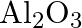 and
and 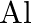 can reduce
can reduce 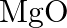 ? What are those conditions?
? What are those conditions?
Is it true that under certain conditions, Mg can reduce 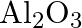 and
and 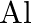 can reduce
can reduce 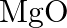 ? What are those conditions?
? What are those conditions?
