Ans: It is given that ![]() is doped with
is doped with ![]() of
of ![]() .
.
This means that ![]() of
of ![]() is doped with
is doped with ![]() of
of ![]() ,
,
Therefore, 1 mol of ![]() is doped with
is doped with ![]() of
of ![]()
If 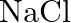 is doped with
is doped with 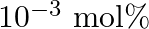 of
of 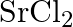 , what is the concentration of cation vacancies?
, what is the concentration of cation vacancies?
If 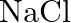 is doped with
is doped with 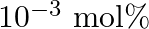 of
of 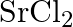 , what is the concentration of cation vacancies?
, what is the concentration of cation vacancies?
