Ans: We are given the overall stability constant ![]() .
.
The overall complex dissociation equilibrium constant is the reciprocal of the overall stability constant. This is given below:
Overall dissociation constant ![]()
Calculate the overall complex dissociation equilibrium constant for the 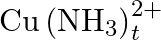 ion, given that
ion, given that 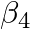 for this complex is
for this complex is 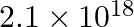 .
.
Calculate the overall complex dissociation equilibrium constant for the 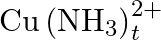 ion, given that
ion, given that 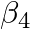 for this complex is
for this complex is 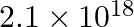 .
.
