Chloride radical is detected by the chromyl chloride test. In this test, chromyl chloride gas (orange red color) is produced. Equation Involved – 4NaCl + K2Cr2O7 + 6H2SO4 → 4NaHSO4 + 2KHSO4 + 3H2O +...
Test
What do you understand by lanthanide contraction
The lanthanide contraction is the decrease in the atomic or ionic radii with increase in the atomic number of lanthanides
What are lanthanide elements?
Lanthanide elements resembles a lot in properties with lanthanum. Lanthanide is group of 14 elements from atomic number 58 to 71. In these elements on increasing atomic number electron enters into...
Explain oxidization properties of potassium permanganate in acidic medium.
2KMnO4 + 8H2SO4 + 10KI → 6K2SO4 + 8H2O + 5I2 2KMnO4 + 5SO2 + 2H2O → 2MnSO4 + 2H2SO4 + K2SO4 2KmO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2 5COOH – COOH + [5O] → 10CO2 + 5H2O
Give two differences between double salt and complex salt.
Answer: Double salt Complex salt A double salt is a combination of two salt compounds. A complex salt is a molecular structure that is composed of one or more complex ions. Double salts can give...
Give two differences between DNA and RNA.
Answer: DNA RNA DNA – Deoxyribo Nucleic Acid RNA – Ribo Nucleic acid DNA consists of adenine (A), cytosine (C), guanine (G), and thymine (T) RNA consists of adenine (A), cytosine (C), guanine (G),...
Which of the following compounds has tetrahedral geometry? (a) ![Rendered by QuickLaTeX.com \left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{-2}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c5a91864ba7e1e0ed1b5d273d822f5e5_l3.png) (b)
(b) ![Rendered by QuickLaTeX.com \left[\mathrm{Pd}(\mathrm{CN})_{4}\right]^{2-}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ffda616d3dbe7dc8925722e87005924e_l3.png) (c)
(c) ![Rendered by QuickLaTeX.com \left[\mathrm{PdCl}_{4}\right]^{2-}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-90cc6a4f4d42dee46e06d6f8ed516b7a_l3.png) (d)
(d) ![Rendered by QuickLaTeX.com \left[\mathrm{NiCl}_{4}\right]^{2}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-149ee5c95bd9cfcc4405704fba2e1bab_l3.png)
SOL: Correct option is D. $\left[\mathrm{NiCl}_{4}\right]^{2}$
Oxidation number of gold metal is (a)+1 (b) 0 (c) 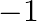 (d) all of these
(d) all of these
Sol: Correct option is B. 0
Match the example given in Column I with the name of the reaction in Column II
Solution: (i) is e (ii) is d (iii) is a (iv) is b (v) is f (vi) is c
Match the reactions given in Column I with the suitable reagents given in Column II.
Solution: (i) is c (ii) is d (iii) is a (iv) is b
Match the acids given in Column I with their correct IUPAC names given in Column II.
Solution: (i) is b (ii) is e (iii) is d (iv) is a (v) is c
Match the common names given in Column I with the IUPAC names given in Column II
Solution: (i) is d (ii) is e (iii) is a (iv) is b (v) is c
Can Gatterman-Koch reaction be considered similar to Friedel Craft’s acylation? Discuss.
Solution: Both reactions resemble each other. In Friedel Craft’s acylation reaction, an aryl group or benzene is treated with an acid chloride in the presence of anhydrous AlCl3 and corresponding...
Ethylbenzene is generally prepared by acetylation of benzene followed by reduction and not by direct alkylation. Think of a possible reason.
Solution: This is due to the formation of polysubstituted products. To avoid the formation of polysubstituted products Friedel-craft’s alkylation reaction is not used for the preparation of...
Complete the following reaction sequence.
Solution:
Why are carboxylic acids more acidic than alcohols or phenols although all of them have a hydrogen atom attached to an oxygen atom (—O—H)?
Solution: Due to the resonance in carboxylic acids, the negative charge is at the more electronegative oxygen whereas, in alcohols or phenols, the negative charge is on a less electronegative atom....
. Identify the compounds A, B and C in the following reaction.
Solution: Compound A = CH3-MgBr Compound B = CH3-COOH Compound C = CH3COOCH3
Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
Solution: The oxygen atom in carbonyl compound pull more shared pair of electron towards itself and so, carbon acquires partial positive charge and oxygen acquires partial negative charge in...
Alkenes and carbonyl compounds both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
Solution: Both the compounds carbon atom is attached to the electronegative atom oxygen. Thus the oxygen pulls more shared pair of electron towards them and a partial positive charge will be...
Arrange the following in decreasing order of their acidic strength. Explain the arrangement. C6H5COOH, FCH2COOH, NO2CH2COOH
Solution: NO2CH2COOH > FCH2COOH > C6H5COOH. NO2CH2COOH is most acidic among the given three compounds. Electron withdrawing groups like -NO2, increases the acidity of carboxylic acids by...
Compound ‘A’ was prepared by oxidation of compound ‘B’ with alkaline KMnO4. Compound ‘A’ on reduction with lithium aluminium hydride gets converted back to compound ‘B’. When compound ‘A’ is heated with compound B in the presence of H2SO4 it produces the fruity smell of compound C to which family the compounds ‘A’, ‘B’ and ‘C’ belong to?
Solution: Compound ‘A’ belongs to the carboxylic acid. Compound ‘B’ belongs to alcohol. Compound ‘C’ belongs to an ester group.
What product will be formed on reaction of propanal with 2-methyl propanal in the presence of NaOH? What products will be formed? Write the name of the reaction also.
Solution: When propanal reacts with 2-methyl propanal in the presence of NaOH, the mixture of aldehydes are formed. Both the reactants have an alpha-hydrogen and hence, can undergo cross aldol...
Arrange the following in decreasing order of their acidic strength and give the reason for your answer.
Solution: FCH2COOH > ClCH2COOH > C6H5CH2COOH > CH3COOH > CH3CH2OH. CH3CH2OH is least acidic among the given compounds. C6H5CH2COOH is more acidic than CH3COOH due to the resonance in...
Oxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
Solution: Solution: The products formed on oxidation of 2, 5-dimethylhexan-3-one are the mixtures of ketone and carboxylic acids. Ketone is then further oxidized to carboxylic acids. Overall the...
Name the electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous AlCl3. Name the reaction also.
Solution: The electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous AlCl3 is benzoylinium cation. The product formed in this reaction is benzophenone....
Benzaldehyde can be obtained from benzal chloride. Write reactions for obtaining benzyl chloride and then benzaldehyde from it.
SOLUTION: Toluene is first converted to benzal chloride by side-chain chlorination, in presence of Chlorine gas and light. Benzal chloride on hydrolysis at 373K gives benzaldehyde.
Benzaldehyde can be obtained from benzal chloride. Write reactions for obtaining benzyl chloride and then benzaldehyde from it.
Solution:
Write IUPAC names of the following structures.
Solution: (i) Ethane-1,2-dial. (ii) Benzene-1, 4-dicarbaldehyde. (iii) 3-Bromobenzaldehyde.
Give the structure of the following compounds. (i) 4-Nitropropiophenone (ii) 2-Hydroxycyclopentanecarbaldehyde (iii) Phenyl acetaldehyde
Give the IUPAC names of the following compounds
Solution: (i) 3-Phenylprop-2-ene-1-al. (ii) Cyclohexanecarbaldehyde (iii) 3-Oxopentan-1-al (iv) IUPAC name: But-2-enal
Arrange the bonds in order of increasing ionic character in the molecules: LiF, 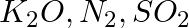 , and
, and 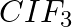 .
.
Solution: The difference in electronegativity between constituent atoms determines the ionic character of a molecule. As a result, the greater the difference, the greater the ionic character of a...
Explain with the help of suitable example polar covalent bond.
Solution: The bond pair of electrons are not shared equally when two unique atoms with different electronegativities join to form a covalent bond. The bond pair is attracted to the nucleus of an...
Define electronegativity. How does it differ from electron gain enthalpy?
Solution: "Electronegativity refers to an atom's ability to attract a bond pair of electrons towards itself in a chemical compound." Sr. No Electronegativity Electron affinity 1 A tendency to...
Write the significance/applications of dipole moment.
Solution: There is a difference in electro-negativities of constituents of the atom in a heteronuclear molecule, which causes polarisation. As a result, one end gains a positive charge, while the...
Although both 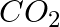 and
and 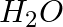 are triatomic molecules, the shape of the
are triatomic molecules, the shape of the 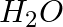 molecule is bent while that of
molecule is bent while that of 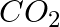 is linear. Explain this on the basis of dipole moment.
is linear. Explain this on the basis of dipole moment.
Solution: $CO_2$ has a dipole moment of 0 according to experimental results. And it's only possible if the molecule's shape is linear, because the dipole moments of the C-O bond are equal and...
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :(iii) Al and N.
Solution: Below is a list of Lewis symbols. To form a cation, a metal atom loses one or more electrons, while a nonmetal atom gains one or more electrons. Ionic bonds are formed between cations and...
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions : (i) K and S (ii) Ca and O
Solution: Below is a list of Lewis symbols. To form a cation, a metal atom loses one or more electrons, while a nonmetal atom gains one or more electrons. Ionic bonds are formed between cations and...
Temperature dependence of resistivity ρ(T) of semiconductors, insulators, and metals is significantly based on the following factors:
a) number of charge carriers can change with temperature T
b) time interval between two successive collisions can depend on T
c) length of material can be a function of T
d) mass of carriers is a function of T
The correct answer is a) number of charge carriers can change with temperature T b) time interval between two successive collisions can depend on T
Write the resonance structures for  , and
, and 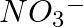
Solution: Resonance is the phenomenon that allows a molecule to be expressed in multiple ways, none of which fully explain the molecule's properties. The molecule's structure is called a resonance...
Explain the important aspects of resonance with reference to the 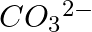 ion.
ion.
Solution: However, while the carbonate ion cannot be represented by a single structure, the properties of the ion can be described by two or more different resonance structures. The actual structure...
Define Bond length.
Solution: Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
How do you express the bond strength in terms of bond order?
Solution: During the formation of a molecule, the extent of bonding that occurs between two atoms is represented by the bond strength of the molecule. As the bond strength increases, the bond...
Discuss the shape of the following molecules using the VSEPR model: 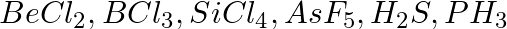
Solution: $BeCl_2$ The central atom does not have a lone pair, but it does have two bond pairs. As a result, its shape is AB2, or linear. $BCl_3$ The central atom has three bond pairs but no lone...
Define the octet rule. Write its significance and limitations
Solution: “Atoms can combine either by transferring valence electrons from one atom to another or by sharing their valence electrons in order to achieve the closest inert gas configuration by having...
Write Lewis symbols for the following atoms and ions: Sand 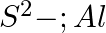 and
and 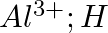 and
and 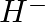
Solution: For S and S2- A sulphur atom has only 6 valence electrons, which is a very small number. As a result, the Lewis dot symbol for the letter S is The presence of a...
Write Lewis dot symbols for atoms of the following elements :c) B d) O
Solution: Boron atoms have only three valence electrons, which is a very small number. As a result, the Lewis dot symbols for B are as follows: The oxygen atom has only six valence...
Write a test to differentiate between pentan-2-one and pentan-3-one.
Solution: One can differentiate between pentan-2-one and pentan-3-one by iodoform test. Pentan-2-one have a –CO-CH3 group and therefore forms a yellow precipitate of Iodoform. Pentan-2-one gives a...
Why is there a large difference in the boiling points of butanal and butane-1-ol?
Solution: Butanal has no intermolecular hydrogen bonding but butan-1-ol has intermolecular hydrogen bonding. This bonding in butan-1-ol makes it more stable at a higher temperature than butanal.
Which of the following is the correct representation for intermediate of nucleophilic addition reaction to the given carbonyl compound (A) :
Solution: Option (A) and (B) are the answers. Reason:
Benzophenone can be obtained by ____________. (i) Benzoyl chloride + Benzene + AlCl3 (ii) Benzoyl chloride + Diphenyl cadmium (iii) Benzoyl chloride + Phenyl magnesium chloride (iv) Benzene + Carbon monoxide + ZnCl2
Solution: Option (i) and (ii) are the answers Reason: Benzophenone can be obtained by the Friedel-Craft acylation reaction. The reaction is shown as
Through which of the number of the following reactions of carbon atoms can be increased in the chain? (i) Grignard reaction (ii) Cannizaro’s reaction (iii) Aldol condensation (iv) HVZ reaction
Solution: Option (i) and (iii) are the answers. Reason: Grigned reaction and aldol condensation is used to increase the number of carbon attom in the chain as follows:
Write Lewis dot symbols for atoms of the following elements :
a) Mg
b) Na
Solution: Only two valence electrons exist in the magnesium atom. As a result, the Lewis dot symbols for Mg are as follows: Only one valence electron exists in the sodium atom. As a...
Which of the following conversions can be carried out by Clemmensen Reduction? (i) Benzaldehyde into benzyl alcohol (ii) Cyclohexanone into cyclohexane (iii) Benzoyl chloride into benzaldehyde (iv) Benzophenone into diphenylmethane
Solution: Option (ii) and (iv) are the answers. Reason: The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid...
Explain the formation of a chemical bond.
Answer: "A chemical bond is an attractive force that holds a chemical species' constituents together." For chemical bond formation, many theories have been proposed, including valence shell electron...
Treatment of compound with NaOH solution yields(i) Phenol (ii) Sodium phenoxide (iii) Sodium benzoate (iv) Benzophenone
Solution: Option (ii) and (iii) are the answers. Reason: Treatment of compound with NaOH yields sodium phenoxide and sodium by means of nucleophilic substitution reaction as follows
13. Which of the following compounds do not undergo aldol condensation?
Solution: Option (ii) and (iv) are the answers. reason: Aldehydes and ketones and having at least one alpha-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to beta-hydroxy...
In Clemmensen Reduction carbonyl compound is treated with _____________. (i) Zinc amalgam + HCl (ii) Sodium amalgam + HCl (iii) Zinc amalgam + nitric acid (iv) Sodium amalgam + HNO3
Solution: Option (i) is the answer. Reason: From the above reaction carbonyl group is treated with Zn−Hg(Zinc Amalgum) and HCl
Which of the following compounds will give butanone on oxidation with alkaline KMnO4 solution? (i) Butan-1-ol (ii) Butan-2-ol (iii) Both of these (iv) None of these
Solution: Option (ii) is the answer.
Which is the most suitable reagent for the following conversion?(i) Tollen’s reagent (ii) Benzoyl peroxide (iii) I2 and NaOH solution (iv) Sn and NaOH solution
Solution: Option (iii) is the answer. Reason: This reaction is called as lodoform reaction.
Compound A and C in the following reaction are :_____________
Solution: Option (ii) is the answer. Reason:
Structure of ‘A’ and type of isomerism in the above reaction are respectively. (i) Prop–1–en–2–ol, metamerism (ii) Prop-1-en-1-ol, tautomerism (iii) Prop-2-en-2-ol, geometrical isomerism (iv) Prop-1-en-2-ol, tautomerism
Solution: Option (iv) is the answer. reason: Structure of A and the type of isomerism in the above reaction are Prop-1-en-2-ol, tautomerism respectively. Enol form tautomerises into keto...
Which product is formed when the compoundis treated with concentrated aqueous KOH solution?
Solution: Option (ii) is the answer. Reason: Benzaldhyde C6H5CHO on treatment with KOH yields the corresponding alcohol and acid. In this reaction, there is no alpha hydrogen atom present in...
Cannizaro’s reaction is not given by _____________.
Solution: Option (iv) is the answer. Reason: CH3CHO will not give Cannizzaro’s reaction because it contains a-hydrogen while other three compounds have no a-hydrogen. Hence, they will give...
The reagent which does not react with both, acetone and benzaldehyde. (i) Sodium hydrogen sulphite (ii) Phenyl hydrazine (iii) Fehling’s solution (iv) Grignard reagent
Solution: Option (iii) is the answer. Reason: Aromatic aldehydes and ketones does not respond to Fehling's test. Sodium hydrogen sulphite,phenyl hydrazine, grignard reaction are common for carbonyl...
Compound can be prepared by the reaction of _____________.
Solution: Option (ii) is the answer. Reason:
The correct order of increasing acidic strength is _____________. (i) Phenol < Ethanol < Chloroacetic acid < Acetic acid (ii) Ethanol < Phenol < Chloroacetic acid < Acetic acid (iii) Ethanol < Phenol < Acetic acid < Chloroacetic acid (iv) Chloroacetic acid < Acetic acid < Phenol < Ethanol
Solution: Option (iii) is the answer. Reason: The correct order of increasing acidic strength is Ethanol < Phenol < Acetic acid < Chloroacetic acid. Phenol is more acidic than ethanol...
Which of the following compounds is most reactive towards nucleophilic addition reactions?
Solution: Option (i) is the answer.
Addition of water to alkynes occurs in acidic medium and the presence of Hg2+ ions as a catalyst. Which of the following products will be formed on addition of water to but-1-one under these conditions.
Solution: Option (ii) is the answer. Reason: Addition of water to but-1-yne in the presence of H2SO4 and HgSO4 gives 2-butaone. The addition takes place by markovnikoff's rule....
Arrange the following compounds in increasing order of dipole moment. CH3CH2CH3, CH3CH2NH2, CH3CH2OH
Solution: CH3CH2CH3 < CH3CH2NH2 < CH3CH2OH The dipole moment of CH3CH2OH is greater than that of CH3CH2NH2. CH3CH2CH3 has the least dipole moment among the three given compounds because it is...
Predict the product of the reaction of aniline with bromine in a non-polar solvent such as CS2.
Solution: The products formed in the reaction of aniline with bromine in a non-polar solvent such as CS2 are 4-Bromoaniline and 2-Bromoaniline where 4-Bromoaniline is the major product.
Under what reaction conditions (acidic/basic), the coupling reaction of aryldiazonium chloride with aniline is carried out?
Solution: This reaction is carried out in a mild basic medium. This is an electrophilic substitution reaction. Aryldiazonium chloride reacts with aniline to form a yellow dye of p-Aminoazobenzene.
Explain why MeNH2 is a stronger base than MeOH?
Solution: MeNH2 is a stronger base than MeOH because of the lower electronegativity and the presence of the lone pair of electrons on the nitrogen atom in MeNH2.
Why is benzene diazonium chloride not stored and is used immediately after its preparation?
Solution: At high temperatures, benzene diazonium chloride is highly soluble in water, and at low temperatures, it is a very stable compound in water. Because it is unstable, it should be used as...
What is the best reagent to convert nitrile to primary amine?
Solution: LiAlH4 and Sodium/Alcohol are the best reagents for converting nitrile to primary amine. The nitriles can be converted into a corresponding primary amine through reduction.
Why is NH2 group of aniline acetylated before carrying out nitration?
Solution: The NH2 group of aniline is acetylated before nitration to control the nitration reaction and the formation of tarry oxidation products and nitro derivatives. P-nitroaniline is the main...
Which of the following reactions belong to electrophilic aromatic substitution?
(i) Bromination of acetanilide
(ii) Coupling reaction of aryldiazonium salts
(iii) Diazotisation of aniline
(iv) Acylation of aniline
Solution: Option (i) and (ii) are the answers. Reason:...
Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product?
(i) Acetyl chloride/pyridine followed by reaction with conc. H2SO4 + conc. HNO3
(ii) Acetic anhydride/pyridine followed by conc. H2SO4 + conc. HNO3
(iii) Dil. HCl followed by reaction with conc. H2SO4 + conc. HNO3
(iv) Reaction with conc. HNO3 + conc.H2SO4
Solution: Option (i) and (ii) are the answers. Reason: In addition to the nitro derivatives, direct nitration of aniline produces tarry oxidation products. Furthermore, in a strongly acidic...
Which of the following reactions are correct?
Solution: Option (i) and (iii) are the answers. Reason:
Which of the following amines can be prepared by Gabriel synthesis.
(i) Isobutyl amine
(ii) 2-Phenylethylamine
(iii) N-methyl benzylamine
(iv) Aniline
Solution: Option (i) and (ii) are the answers. Reason: Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium...
Arenium ion involved in the bromination of aniline is __________.
Solution: Option (i), (ii) and (iii) are the answers. Reason: Arenium ion involved in the bromination of aniline are as follows:
The product of the following reaction is __________.
Solution: Option (A) and (B) is the answer. Reason:
The reagents that can be used to convert benzene diazonium chloride to benzene are __________.
(i) 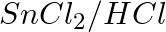
(ii) 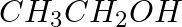
(iii) 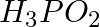
(iv) 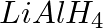
Solution: Option (ii) and (iii) are the answers. Reason: Hypophosphorous acid (phosphinic acid) and ethanol, for example, reduce diazonium salts to arenes, which are then oxidised to phosphorous...
Which of the following species are involved in the carbylamine test?
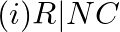
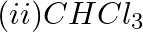
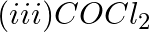
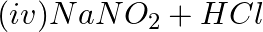
Solution: Option (i) and (ii) are the answers. Reason: Only RNC and CHCl3 are involved in carbylamine reaction.
Reduction of nitrobenzene by which of the following reagent gives aniline?
(i) Sn/HCl
(ii) Fe/HCl
(iii) H2-Pd
(iv) Sn/NH4OH
Solution: Option (i), (ii), and (iii) are the answers. Reason: They are reducing agents.
Which of the following cannot be prepared by Sandmeyer’s reaction?
(i) Chlorobenzene
(ii) Bromobenzene
(iii) Iodobenzene
(iv) Fluorobenzene
Solution: Option (iii) and (iv) are the answers. Reason: Sandmeyer's reaction is used for the preparation of chlorobenzene and bromobenzene.
Which of the following methods of preparation of amines will not give the same number of carbon atoms in the chain of amines as in the reactant?
(i) The reaction of nitrite with LiAlH4.
(ii) The reaction of the amide with LiAlH4 followed by treatment with water.
(iii) Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis.
(iv) Treatment of amide with bromine in the aqueous solution of sodium hydroxide.
Solution: Option (iv) is the answer. Reason: In Hoffmann Bromide degradation as the word, suggest, the amide is reduced to an amine with 1- carbonless, so this is the method in which we don't get...
Which of the following should be most volatile?
Solution: Option (ii) is the answer. Reason: The order of boiling points of isomeric amines is 1 amine > 2 amines > 3 amines. 3 amines have no intermolecular association because there are no H...
Among the following amines, the strongest Brönsted base is __________.
Solution; Option (iv) is the answer. Reason: Option (iv) is the strongest Bronsted base as there is no delocalization of lone pair of electron of the atom which is not possible in aniline and in...
The correct decreasing order of basic strength of the following species is _______. H2O, NH3, OH–, NH2– (i) NH2– > OH – > NH3 > H2O (ii) OH– > NH2– > H2O > NH3 (iii) NH3 > H2O > NH2– > OH– (iv) H2O > NH3> OH– > NH2–
Solution: Option (i) is the answer. Reason: NH3 is more basic than H2O, therefore NH2− (Conjugate base of weak acid NH3) is a stronger base than OH−.
Among the following amines, the strongest Brönsted base is __________.
Solution; Option (iv) is the answer. Reason: Option(iv)is the strongest Bronsted base as there is no delocalisation of lone pair of electron of N atom which is not possible in aniline and in...
Which of the following compounds is the weakest Brönsted base?
Solution: Option (iii) is the answer. Reason: A Bronsted Lowry base is a proton acceptor or hydrogen ion acceptor. Amines have a stronger tendency to accept protons and are strong Bronsted bases....
Which of the following compound will not undergo an azo coupling reaction with benzene diazonium chloride.
(i) Aniline
(ii) Phenol
(iii) Anisole
(iv) Nitrobenzene
Solution: Option (iv) is the answer. Reason: Diazonium cation is a weak electrophile and hence it reacts with electron-rich compounds containing electron-donating groups such as −OH, -$NH_2$ and...
The best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is
(i) Hoffmann Bromamide reaction
(ii) Gabriel phthalimide synthesis
(iii) Sandmeyer reaction
(iv) Reaction with 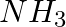
Solution: Option (ii) is the answer. Reason: Best method for preparing primary aminos form alkyl halides without changing the number of carbon atoms in the chain is Gabriel synthesis. Because this...
The reaction Ar + N2Cl– → (Cu/HCl)– ArCl + N2 + CuCl is named as _________.
(i) Sandmeyer reaction
(ii) Gatterman reaction
(iii) Claisen reaction
(iv) Carbylamine reaction
Solution: Option (ii) is the answer. Reason: Diazonium salts in the presence of copper powder and halogen acid give aryl halide. Gattermann reaction is a variation of Sandmeyer reaction in which...
Acid anhydrides on reaction with primary amines give ____________.
(i) amide
(ii) imide
(iii) secondary amine
(iv) imine
Solution: Option (i) is the answer Reason: When acid anhydrides react with primary amines, they produce amide. The H atom of the amino group is replaced with an acyl group in this nucleophilic...
The most reactive amine towards dilute hydrochloric acid is ___________.
Solution: Option (ii) is the answer. Reason: The reactivity of amines is proportional to their basicity. If the R group is, the order of basicity is secondary amine ...
Reduction of aromatic nitro compounds using Fe and HCl gives __________.
(i) aromatic oxime
(ii) aromatic hydrocarbon
(iii) aromatic primary amine
(iv) aromatic amide
Solution: Option (iii) is the answer. Reason: Reduction of nitro aryl compounds in presence of Fe and HCl gives aromatic primary amines.
In the nitration of benzene using a mixture of conc. 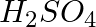 and conc.
and conc. 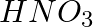 ,the species which initiates the reaction is __________.
,the species which initiates the reaction is __________.
(i) 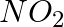
(ii) 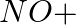
(iii) 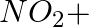
(iv) 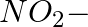
Solution: Option (iii) is the answer. Reason:
The gas evolved when methylamine reacts with nitrous acid is __________.
(i) 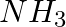 (ii)
(ii) 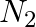 (iii)
(iii) 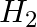 (iv)
(iv) 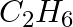
Solution: Option (ii) is the answer. Reason:
Methylamine reacts with HNO2 to form _________.
(i) 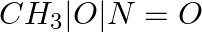 (ii)
(ii) 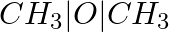 (iii)
(iii) 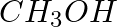 (iv)
(iv) 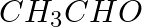
Solution: Option (iii) is the answer. Reason:
The correct increasing order of basic strength for the following compounds is _________.
(i) II < III < I
(ii) III < I < II
(iii) III < II < I
(iv) II < I < III
Solution: Option (iv) is the answer. Reason: Electron donating: group increases the basicity while electron-withdrawing group decreases the basicity of...
Hoffmann Bromamide Degradation reaction is shown by __________.
(i) 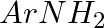
(ii) 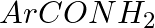
(iii) 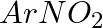
(iv) 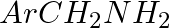
Solution: Option (ii) is the answer. Reason: Where the aryl amide is converted to arylamine in the presence of $Br_2$ and $NaOH$ .
The best reagent for converting 2–phenylpropanamide into 2-phenylpropanolamine is _____.
(i) excess H2 (ii) Br2 in aqueous NaOH (iii) iodine in the presence of red phosphorus (iv) LiAlH4 in ether
Solution: Option (iv) is the answer. Reason:
Amongst the given set of reactants, the most appropriate for preparing 2° amine is _____.
(i) 2° R—Br + NH3
(ii) 2° R—Br + NaCN followed by 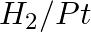
(iii) 1° R—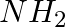 + RCHO followed by %H_2/Pt
+ RCHO followed by %H_2/Pt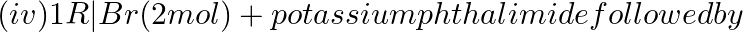 H_3O+$/heat
H_3O+$/heat
Solution: Option (iii) is the answer. Reason:
The source of nitrogen in Gabriel synthesis of amines is _____________.
(i) Sodium azide, NaN3
(ii) Sodium nitrite, NaNO2
(iii) Potassium cyanide, KCN
(iv) Potassium phthalimide
Solution: Option (iv) is the answer. Reason: Gabriel synthesis :The reaction is given to the image.Source of nitrogen atom is Gabriel synthesis is Potassium phthalamide.
To prepare a 1° amine from an alkyl halide with simultaneous addition of one 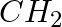 group in the carbon chain, the reagent used as a source of nitrogen is ___________.
group in the carbon chain, the reagent used as a source of nitrogen is ___________.
(i) Sodium amide, NaNH2
(ii) Sodium azide, NaN3
(iii) Potassium cyanide, KCN
(iv) Potassium phthalimide
Solution: Option (iii) is the answer. Reason:
6. Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine? (i) H2 (excess)/Pt (ii) LiAlH4 in ether (iii) Fe and HCl (iv) Sn and HCl
Solution: Option (ii) is the answer. Reason: LiAlH4/ether reduces aryl nitro compounds to azo compounds 2C6H5NO2→LiAIH4C6H5N=N-C6H5
5. Benzylamine may be alkylated as shown in the following equation : C6H5CH2NH2 + R—X → C6H5CH2NHR Which of the following alkyl halides is best suited for this reaction through SN1 mechanism? (i) CH3Br (ii) C6H5Br (iii) C6H5CH2Br (iv) C2H5 Br
Solution: Option (iii) is the answer. Reason: C6H5CH2Br is best suited for this reaction through SN1 mechanism as the carbocation (C6H5CH2) formed is resonance...
Which of the following is the weakest Brönsted base?
Solution: Option (A) is the answer. Reason: Aniline is the weakest Bronsted base due to delocalization of lone pair of electron...
Amongst the following, the strongest base in aqueous medium is ____________.
(i) 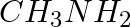
(ii) 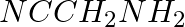
(iii) 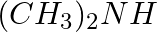
(iv) 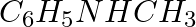
Solution: Option (iii) is the answer. Reason: Due to the electron releasing nature of the alkyl group, it (R) pushes electrons towards nitrogen and thus makes the uncharged electrons pair available...
The correct IUPAC name for 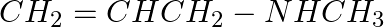 is (i) Allylmethylamine (ii) 2-amino-4-pentene (iii) 4-aminopent-1-ene (iv) N-methylprop-2-en-1-amine
is (i) Allylmethylamine (ii) 2-amino-4-pentene (iii) 4-aminopent-1-ene (iv) N-methylprop-2-en-1-amine
Solution: Option (iv) is the answer. Reason: $CH_2=CHCH_2-NHCH_3$ N−methylprop−2−en−1−amine.
Which of the following is a 3° amine?(i) 1-methylcyclohexylamine (ii) Triethylamine (iii) tert-butylamine (iv) N-methyl aniline
Solution: Option (ii) is the answer. Reason: Triethylamine is a 3° amine because of amonia in which each hydrogen atom is substituted by an methyl group.
Which of the following reaction does NOT yield an amine
(1) (2) (3) (4) Correct Answer: Option (4) Explanation: The given reaction in the fourth option does not yield an amine whereas the rest of the reactions...
Identity ‘Z’ in the following series of reaction
1.Butan-1-ol 2.2-chlorobutane 3.Butan-2-ol 4.But-2-ene Correct Answer: 3.Butan-2-ol Explanation: Identification of Z - Butan-2-ol
Which of the following compounds is obtained when t-butyl bromide is treated with alcoholic ammonia?
(1) (2) (3) (4) Correct Answer: Option (3) Explanation:
Identify the product Y in the following reaction
1.Gluconic acid 2.Saccharic acid 3.n-Hexane 4.Glucoxime Correct Answer: 2. Saccharic acid Explanation: Identification of Y - Saccharic acid
Identify ‘A’ in the following reaction
1. 2.R-NH-OH 3.R-COOH 4.R-NH2 Correct Answer: 2.R-NH-OH Explanation: Identification of A - R-NH-OH
Which among the following statements about terpenes is NOT true?
1.Terpenes occur in essential oils 2.Terpenes include vitamin A, E and K 3.Terpenes consist of isoprene units 4.Terpenes are saturated hydrocarbons Correct Answer: 4. Terpenes are saturated...
How many pi bonds and sigma bound are present in following molecule?
1.5 pi,14 sigma - bonds 2.3 pi,17 sigma - bonds 3.3 pi, 17 sigma - bonds 4.2 pi, sigma - bonds Correct Answer: 3.3 pi, 17 sigma - bonds Explanation: 3 pi, 17 sigma - bonds are...
Aluminium crystallizes in face centered cubic structure, having atomic radius 125pm. The edge length of unit cell of aluminium is
253.5 pm 353.5 pm 465 pm 250 pm Solution: 353.5 pm $ For\text{ }a~FCC~or~~CCP~unit\text{ }cell,we\,have: $ $ 4r=\surd 2\times a $ $ \Rightarrow a=\frac{4r}{\surd 2}=353.5pm $
Which of the following is Rosenmund reduction?
(1) (2) (3) (4) Correct Answer: (3) Explanation: Rosenmund reduction reaction -
Identify the correct statement from the following :
(1) Blister copper has blistered appearance due to evolution of CO2.
(2) Vapour phase refining is carried out for Nickel by Van Arkel method.
(3) Pig iron can be moulded into a variety of shapes.
(4) Wrought iron is impure iron with 4% carbon.
Correct option: (3) Explanation: Because of the evolution of $SO_2$, not $CO_2$, blister copper has a blistered appearance. The Van Arkel method is used to obtain ultra-pure Titanium through vapor...
An alkene on ozonolysis gives methanal as one of the product. Its structure is
Correct option: (2) On ozonolysis, the structure in option B produces methane, as shown in the reaction above.
When carbolic acid is heated with concentrated nitric acid in presence of concentrated sulphuric acid it forms
1.benzoic acid 2.picric acid 3.phthalic acid 4.benzene sulphonic acid Correct Answer: 2. picric acid Explanation: When carbolic acid is heated with concentrated nitric acid in presence of...
For the following reaction, what is the value of ∆S(total) at 298k?
Fe2O3(s) + 3 CO(g) 2Fe(s) + 3CO2(g) o = -29.8 KJ and So =15JK-1. 1.29.8 J 2.100.0 J 3.298.0J 4.115.OJ Correct Answer: 4.115.OJ Explanation: The value of ∆S(total) at 298k is...
What is the oxidation number of carbons in glucose?
1.-6 2.+6 3.+3 4.Zero Correct Answer: 4. Zero Explanation: Zero is the oxidation number of carbons in glucose.
The rate of constant for a second order reaction A→product is 1.62M-1s-1. What will be the rate of reaction when concentration of reactant is 2 x 10-3M?
1.3.24 x 10-3Ms-1 2.3.24 x 10-6 Ms-1 3.6.48 x 10-6 Ms-1 4.2 x 10-3 Ms-1 Correct Answer: 3.6.48 x 10-6 Ms-1 Explanation: The rate of reaction when concentration of reactant is 2 x 10-3M is...
Calcite crystals used in Nicol’s prism are formed of
1.CaC2 2.CaCO3 3.CaCL2 4.CaO Correct Answer: 2.CaCO3 Explanation: Calcite crystals used in Nicol's prism are formed of CaCO3
H2 molecule is more stable than Li2 molecule, because
1.in H2 molecule 15 molecular orbitals are shielded by electrons are shielded by electrons. 2.In H2 bond order is one. 3.In Li2 molecule 15 molecular orbitals are shielded by electrons. 4.In Li2...
Which of the following monomers is used in manufacture of Neoprene rubber?
1.1,3-Butadien 2.styrene 3.2-chlorobuta-1,3-diene 4.Isobutylene Correct Answer: 3.2-chlorobuta-1,3-diene Explanation: 2-chlorobuta-1,3-diene monomer is used in the manufacture of Neoprene...
The unit of atomic mass, amu is replaced by u, here u stands for
1.unified mass 2.united mass 3.unique mass 4.universal mass Correct Answer: 1. unified mass Explanation: u stands for unified mass.
What is the lowest oxidation state possessed by phosphorus in its oxyacid?
1.+4 2.+2 3.+5 4.+1 Correct Answer: 4. +1 Explanation: +1 is the lowest oxidation state possessed by phosphorus in its oxyacid
What happen during bessemerization process of copper from copper pyrites?
1.Au and Ag metals are deposited as anode mud. 2.Impurities as As and Sb are removed as volatile oxides. 3.Cu is obtained by auto reduction of Cu2O and CuS. 4.Iron is removed in the form of slag....
What is the common unit of conductivity if the dimension is expressed in centimeter?
1.Ω cm-1 2.Ω-1 cm-1 3.Ω cm 4.Ω-1 cm Correct Answer: 2. Ω-1 cm-1 Explanation: Ω-1 cm-1 is the common unit of conductivity if the dimension is expressed in centimeter.
Blurring of vision is a side effect caused by the use of
1.antibiotics 2.antacides 3.tranquilizers 4.analgesics Correct Answer: 3. tranquilizers Explanation: Blurring of vision is a side effect caused by the use of tranquillizers....
What is the boiling point of heavy water?
1.100.4oC 2.101.4OC 3.273OC 4.100OC Correct Answer: 2.101.4OC Explanation: 101.4OC is the boiling point of heavy water.
What is effective atomic number of Fe in [Fe(CN)6]4- (At.no. of Fe =26)
1.34 2.26 3.36 4.35 Correct Answer: 3.36 Explanation: 36 is effective atomic number of Fe in [Fe(CN)6]4-
Which among the following elements has lowest density and is lightest?
1.Scandium 2.Cobalt 3.cpper 4.iron Correct Answer: 1. Scandium Explanation: Scandium is the element that has lowest density and is lightest.
What is the value of radius ratio of ionic crystal having coordination number six?
1.Greater than 0.732 2.in between 0.414 to 0.732 3.in between 0.225 to 0.414 4.Less than 0.225 Correct Answer: 1.in between 0.414 to 0.732 Explanation: The value of radius ratio of ionic...
What is the molar conductivity of 0.1 M NaCl its conductivity is 1.06 x 10-2Ω-1cm-1
1.1.06 x 102 Ω-1cm2 mol-1 2.1.06 x 10-2 Ω-1cm2 mol-1 3.9.4 x 10-2 Ω-1cm2 mol-1 4.5.3 x 103 Ω-1cm2 mol-1 Correct Answer: 1.1.06 x 102 Ω-1cm2 mol-1 Explanation: The molar conductivity of 0.1 M...
If a mixture of iodomethane and iodoethane is treated is treated with sodium metal in presence of dry ether it forms
1.popane and ethane 2.ethane and butane 3.propane and butane 4.ethane, propane and butane Correct Answer: 4. ethane, propane and butane Explanation: If a mixture of iodomethane and iodoethane...
Which of the following carbonyl compounds does NOT undergoes aldol condensation?
1.Acetone 2.Benzophenone 3.Acetaldehyde 4.Accetophenone Correct Answer: 2. Benzophenone Explanation: Benzophenone is the carbonyl compound which does not undergoes aldol...
Calculate the number of units cell in the 38.6g of noble having density 19.3gcm-1 and volume of one unit cell is 6.18 x 10-23cm3?
1.3.236 x 1022 2.6.180 x 1023 3.6.236 x 1020 4.3.236 x 1023 Correct Answer: 1.3.236 x 1022 Explanation: 3.236 x 1022 is the number of units cell in the 38.6g of noble having density 19.3gcm-1...
What is the percentage of formaldehyde in formalin?
1.60% 2.40% 3.10% 4.20% Correct Answer: 2.40% Explanation: The percentage of formaldehyde in formalin is 40%.
Which of the following antihistamine contain –CN group?
1.Dimetapp 2.Cimetidine 3.Terfenadine 4.Ranitidine Correct Answer: 2.Cimetidine Explanation: Cimetidine contain –CN group.
Which among the following coordination compounds does not have coordination number equal to number of ligands?
1.[pt(NH3)6]4+ 2.[Co(en)3]3+ 3.[Cu(NH3)4]2+ 4.[Co (NH3)6]3+ Correct Answer: 2.[Co(en)3]3+ Explanation: [Co(en)3]3+does not have coordination number equal to number of...
According to Andrews isothermals at What temperature the carbon dioxide gas starts to condense at 73 atmosphere?
1.21.5oC 2.30.98oC 3.13.1oC 4.48.1oC Correct Answer: 2.30.98o C Explanation: According to Andrews isothermals at 30.98oC the carbon dioxide gas starts to condense at 73...
Sodium crystallizes in bcc structure with radius 1.86 x 10-8cm.What is the edge length of unit cell of sodium?
1.4.3x 10-8 cm 2.3.72 x 10-8 cm 3.7.44 x 10-8 cm 4.5.26 x 10-8 cm Correct Answer: 1.4.3x 10-8 cm Explanation: Sodium crystallizes in bcc structure with radius 1.86 x 10-8cm. The edge length...
Which among the following reactions occurs at the zone of slag formation in extraction of iron by blast furnace?
1.C + ½ O2 → CO 2.CaO + SiO2 → CaSiO3 3.Fe2O3 + 3 CO → 2 Fe + 3CO2 4.Fe2O3 + 3C → 2 Fe + 3 CO Correct Answer: 2. CaO + SiO2 → CaSiO3 Explanation: Reaction Involved - CaO + SiO2...
In the reaction, N2 +3H2 → 2NH3, the rate of disappearance of H2 is 0.002 M/s. The rate of appearance of NH3 is
1.0.0133 M/s 2.0.023 M/s 3.0.004 M/s 4.0.032 M/s Correct Answer: 1.0.0133 M/s Explanation: In the given reaction , the rate of appearance of NH3 is 0.0133 M/s.
Identity the polymer obtained by heating n moles of isobutylene with n moles of isoprene at 1000C in presence of anhydrous AICl3
1.Butyl rubber 2.Buna-N 3.Buna-S 4.Neoprene rubber Correct Answer: 1.Butyl rubber Explanation: Butyl rubber is the polymer which is obtained by heating n moles of isobutylene with n moles of...
Which among the following gas is bubbled through the brine solution during the preparation of sodium carbonate in Solvay’s process?
1.CO2(g) 2.N2(g) 3.NO2(g) 4.O2(g) Correct Answer: 4.O2(g) Explanation: Oxygen gas is bubbled through the brine solution during the preparation of sodium carbonate in Solvay's...
Which among the following elements is a soft element as compared to others
1.CO 2.Zn 3.W 4.Mo Correct Answer: 2.Zn Explanation: Zinc is a soft element when compared to the rest of the elements.
Which of the following changes will changes will cause increase in vapour in vapour pressure of 1 molal aqueous KI solution at same temperature?
1.addition of 0.1 molal solution of NaCl 2.addition of 0.5 molal solution of Na2So4 3.addition of water 4.addition of 1 molal KI solution Correct Answer: 3. addition of water Explanation: By...
In the resonance hybrid of ozone molecule, O-O bond length is
1.128 pm 2.134.5 pm 3.121pm 4.148pm Correct Answer: 1.128 pm Explanation: In the resonance hybrid of ozone molecule, O-O bond length is 128 pm.
In the following reaction, What is the mass of KCL(s) produced ?
2KCLO3(s) 2KCL(s) + 3 O(g) H0 = -78 KJ. if 33.6L of oxygen gas is liberated at S.T.P. (at mass K=39, CL= 35.5 g mol-1) 1.48.0 g 2.7.45 g 3.24.0 g 4.74.5 g Correct Answer: 4.74.5 g...
Enthalpy of fusion of vaporization for water respectively are 6.01 Kj mol-1 and 45.07 kJ mol-1at 0degree C What is enthalpy of sublimation at 0degree C?
1.27.50 KJ mol-1 2.48.07 KJ mol-1 3.51.08 KJ mol-1 4.39.06 KJ mol-1 Correct Answer: 3.51.08 KJ mol-1 Explanation: Enthalpy of fusion of vaporization for water respectively are 6.01 Kj mol-1...
Which of the following statement is NOT correct about solution?
1.The three states of matter solid, liquid and gas may play role of either solute or solvent 2.the component of solution which constitute smaller part is called solute. 3.When water is solvent, the...
The P-P-P bond angle in white phosphorus is
1.90o 2.109o281 3.120o 4.600 Correct Answer: 4.600 Explanation: The P-P-P bond angle in white phosphorus is 4.600
Which of the following pairs of solution is isotonic? (Molar mass urea=60, sucrose=342 g mol-1)
1.3.0 gL-1 urea and 17.19 gL-1 sucrose 2.0.3 gL-1 urea and 1.719 gL-1 sucrose 3.0.gL-1 urea and 1.719 gL-1 sucrose 4.0.3 gL-1 urea and 17.19 gL-1 sucrose Correct Answer: 1.3.0 gL-1 urea and...
When alkyl halide is boiled with large excess of alcoholic ammonia it forms
1.Primary amine 2.tertiary amine 3.secondary amine 4.quatrnary ammonium salt Correct Answer: 1.Primary amine Explanation: When alkyl halide is boiled with large excess of alcoholic ammonia it...
Methoxy ethane on reaction with hot concentrated HI gives
1.iodomethane and ethanol 2.iodomethane and iodoethane 3.methanol and ethanol 4.methanol and iodoethane Correct Answer: 2.iodomethane and iodoethane Explanation: Methoxy ethane on reaction...
Which of the following oxyacid of Sulphur contains S=S linkage?
1.H2S2O4 2.H2SO3 3.H2S2O5 4.H2S2O2 Correct Answer: 4.H2S2O2 Explanation: H2S2O2 contains S=S linkage.
Identity the decreasing order of boiling point of alkanes (1)n-pentane (2) isopentane (3) Neopentane
1.Isopentene ˃ n-pentane ˃ Neopentane 2.Neopentane ˃Isopentane ˃ n-pentane 3.n-pentane ˃ Isopentane ˃ Neopentane 4.Isopentane ˃ Neopentane ˃ n-pentane Correct Answer: 3.n-pentane ˃ Isopentane...
Which of the following is a character of catalyst?
1.it changes the position of equilibrium. 2.it increases the rates of both forward and backward reaction equally in reversible reaction 3.it affects the energies of reactants and products of the...
An increase in the concentration of the reactants of a reaction leads to change in (1) heat of reaction (2) threshold energy (3) collision frequency (4) activation energy
Correct option (1) As the number of molecules per unit volume grows, so does the frequency of collisions.
Match the following and identify the correct option.
Correct option (4) CO(g) + H2(g) = Synthetic gas or water gas Bicarbonates of Ca2+ and Mg2+...
Anisole on cleavage with HI gives
Correct option (4) The methyl(phenyl) oxonium ion is formed when anisole reacts with protons from hydroiodic acid. The reaction is an SN2...
Which of the following oxoacid of sulphur has -O-O- linkage?
(1) 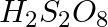 , peroxodisulphuric acid
, peroxodisulphuric acid
(2) 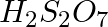 , pyrosulphuric acid
, pyrosulphuric acid
(3) 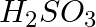 , sulphurous acid
, sulphurous acid
(4)  , sulphuric acid
, sulphuric acid
The correct option is (1) Diagrammatic representation of each compound is as follows,
What is the change in oxidation number of carbon in the following reaction? ![Rendered by QuickLaTeX.com \[CH _{4}( g )+4 Cl _{2}( g ) \rightarrow CCl _{4}( l )+4 HCl ( g )\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-80c7d3f47e40e2ab7490771786cbcebe_l3.png)
(1) 0 to 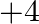
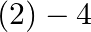 to
to 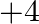
(3) 0 to 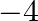
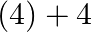 to
to 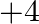
Correct option: (2) The oxidation state carbon of methane is -4 which is the reactant and that on product side is carbon tetrachloride is +4.
The mixture which shows positive deviation from Raoult’s law is
(1) Benzene + Toluene
(2) Acetone + Chloroform
(3) Chloroethane + Bromoethane
(4) Ethanol + Acetone
Correct option (4) Acetone + ethanol deviates from Raoult's law in a positive way. H-bonding exists in pure ethanol, and adding acetone to it causes some H-bonds to break. The observed vapour...
The freezing point depression constant 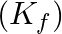 of benzene is
of benzene is 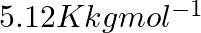 . The freezing point depression for the solution of molality
. The freezing point depression for the solution of molality 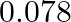 m containing a non-electrolyte solute in benzene is (rounded off upto two decimal places):
m containing a non-electrolyte solute in benzene is (rounded off upto two decimal places):
(1) 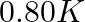 (2)
(2) 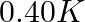 (3)
(3) 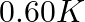 (4)
(4) 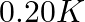
Correct option: (2) As we know that, we evaluate the value using the formula directly, $\begin{aligned} \Delta T _{ f }= i k _{ f } m \\ \Rightarrow \quad \Delta T _{ f } &=1 \times 5.12 \times...
Identify the incorrect statement.
(1) The transition metals and their compounds are known for their catalytic activity due to their ability to adopt multiple oxidation states and to form complexes.
(2) Interstitial compounds are those that are formed when small atoms like 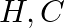 or
or 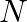 are trapped inside the crystal lattices of metals.
are trapped inside the crystal lattices of metals.
(3) The oxidation states of chromium in 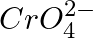 and
and 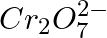 are not the same.
are not the same.
(4) 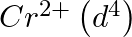 is a stronger reducing agent than
is a stronger reducing agent than 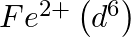 in water.
in water.
Correct option (3) Explanation: Interstitial compounds are those formed when small atoms such as H, B, C, or N are trapped inside metal crystal lattices. Fact: On the basis of standard reduction...
Which of the following equations shows the relationship between heat of reaction at constant pressure and heat of reaction at constant volume if the temperature is not constant?
$ 1.\,\,\Delta H-\Delta n=\Delta URT $ $ 2.\,\,\Delta H-\Delta U=\Delta nRT $ $ 3.\,\,\Delta H=\Delta nRT $ $ 4.\,\,\Delta H=\Delta U-RT $ Solution: $ 2.\,\,\Delta H-\Delta U=\Delta nRT $...
Which of the following elements is refined by zone refining?
A. Gallium B. Bismuth C. Copper D. Zinc Solution: gallium The zone refining method is generally used to refine metalloids and ultra-pure metal is obtained. This is based on the idea that impurities...
Slope of the straight line obtained by plotting 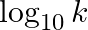 against
against 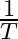 represents what term ? (A)
represents what term ? (A) 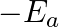 (B)
(B) 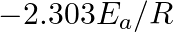 (C)
(C) 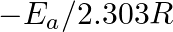 (D)
(D) 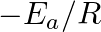
Correct option is C ( $-\mathrm{E}_{\mathrm{a}} / 2.303 \mathrm{R}$) Explanation: The Arrhenius equation is: $ \log _{10} \mathrm{k}=\log _{10} \mathrm{~A}-\frac{\mathrm{E}_{\mathrm{a}}}{2.303...
Calculate the work done during combustion of 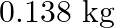 of ethanol,
of ethanol,  at at
at at 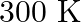 . Given :
. Given : 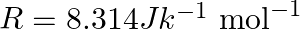 , molar mass of ethanol
, molar mass of ethanol 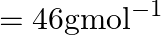 (A)
(A) 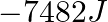 (B)
(B) 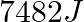 (C)
(C) 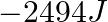 (D)
(D) 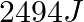
The correct option is B Explanation: The combustion of ethanol, involves the following reaction. $ C_{2} H_{5} O H(l)+3 O_{2}(g) \rightarrow 2 C O_{2}+3 H_{2} O $ Given, Mass of ethanol $=0.138...
With which halogen the reactions of alkanes are explosive ? (A) Fluorine (B) Chlorine (C) Bromine (D) Iodine
Correct answer is A (Fluorine) Explanation: The reactions of alkanes with fluorine are explosive. Fluorine is a highly reactive element exhibiting an exothermic reaction when it reacts with...
What is the geometry of water molecule ? (A) distorted tetrahedral (B) tetrahedral (C) trigonal planer (D) diagonal
Correct option is A Explanation: The tetrahedral geometry of the water molecule is deformed. The electrons in the O atom are divided into two lone pairs and two bond pairs. The sp 3 hybridisation of...
Lactic acid and glycollic acid are the monomers used for preparation of Polymer (A) Nylon2nylon6 (B) Dextron (C) PHBV (D) BunaN
Correct option is B Explanation: Dextron is a biodegradable copolymer of glycolic acid and lactic acid that contains ester linkages.
In case of R, S configuration the group having highest priority is (A) 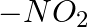 (B)
(B) 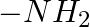 (C)
(C) 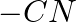
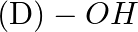
Correct option is D Explanation: The group with the highest priority in the R, S configuration is -OH. The atomic number of O is larger than that of N and C. As a result, oxygen takes priority.
Arenes on treatment with chlorine in presence of ferric chloride as a catalyst undergo what type of reaction ? (A) Electrophilic substitution (B) Nucleophilic substitution (C) Electrophilic addition (D) Nucleophilic addition
Correct option is A Explanation: Arenes undergo halogenation when exposed to chlorine in the presence of a Lewis acid catalyst, ferric chloride, or aluminium chloride, and in the absence of light....
Identify the oxidation states of titanium 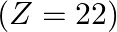 and copper
and copper 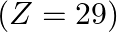 in their colourless compounds. (A)
in their colourless compounds. (A) 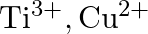 (B)
(B) 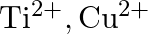 (C)
(C) 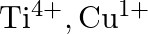 (D)
(D) 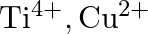
Correct option is C $\mathrm{T} \mathrm{i}^{4+}, \mathrm{Cu}^{+}$ Explanation: The oxidation states of titanium $(\mathrm{Z}=22)$ and copper $(\mathrm{Z}=29)$ in their colourless compounds are...
Identify the monosaccharide containing only one asymmetric carbon atom in its molecule. (A) Ribulose (B) Ribose (C) Erythrose (D) Glyceraldehyde
D is the correct answer Explanation: The monosaccharide glyceraldehyde has only one asymmetric carbon atom in its molecule. An asterisk denotes an asymmetric carbon atom.
Which among the group -15 elements does NOT exists as tetra atomic molecule ? (A) Nitrogen (B) Phosphorus (C) Arsenic (D) Antimony
Correct option is A (Nitrogen) Explanation: Nitrogen does not exist as tetra atomic molecule. It exists as diatomic molecule $N_2$ that can be represented as N≡N.
The correct relation between elevation of boiling point and molar mass of solute is (A) 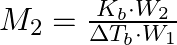 (B)
(B) 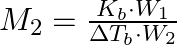 (C)
(C) 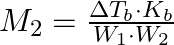 (D)
(D) 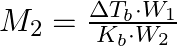
Correct option is (A) $M_{2}=\frac{K_{b} \cdot W_{2}}{\Delta T_{b} \cdot W_{1}}$ EXPLANATION: The correct relation between elevation of boiling point (ΔTb) and molar mass of solute (M2) is...
Which of the following reactions is used to prepare aryl fluorides from diazonium salts and fluoroboric acid ? (A) Sandmeyer reaction (B) BalzSchiemann reaction (C) Gattermann reaction (D) Swarts reaction
Correct option is B Balz-Schiemann reaction EXPLANATION: Balz-Schiemann reaction is used to prepare aryl fluorides from diazonium salts and fluoroboric acid. $ \mathrm{Ar}-\mathrm{N}_{2} \mathrm{X}...
The element that does NOT exhibit allotropy is (A) Phosphorus (B) Arsenic (C) Antimony (D) Bismuth
Correct option is D Explanation: Bismuth is the only element that does not exhibit allotropy. There are two allotropes of nitrogen (solid). There are three types of antimony allotropes. There are...
Conservation of hexane into benzene involves the reaction of (A) Hydration (B) Hydrolysis (C) Hydrogenation (D) Dehydrogenation
Correct option is D Explanation: Conversion of hexane into benzene involves the reaction of dehydrogenation.
Which of the following is NOT a tranquilizer ? (A) Meprobamate (B) Equanil (C) Chlordiazepoxide (D) Bromopheniiramine
Correct option is D Explanation: A tranquillizer is a medicine that is used to treat anxiety, fear, tension, agitation, and other mental problems, with the goal of reducing anxiety and tension....
Which element is obtained in the pure form by van Arkel method ? (A) Aluminium (B) Titanium (C) Silicon (D) Nickel
Correct option is B (Titanium) Explanation: The van Arkel process is used to obtain pure titanium. This procedure can also be used to obtain zirconium. Hall's procedure, Baeyer's process, or...
Which of the following polymers is used to manufacture clothes for firefighters ? (A) Thiokol (B) Kevlar (C) Nomex (D) Dynel
Correct option is C (Nomex) Explanation: Nomex is a synthetic fibre that is used to make clothing for firefighters. It's also utilised to make race car drivers' protective gear. Dynel is a synthetic...
What is the density of solution of sulphuric acid used as an electrolyte in lead accumulator? (A) 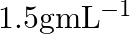 (B)
(B) 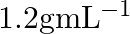 (C)
(C) 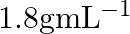 (D)
(D) 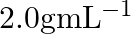
B $1.2 gmL^{-1}$ is the correct answer. Explanation: Sulphuric acid solution used as an electrolyte in a lead accumulator has a density of 1.2 gmL^-1. It is equivalent to 38 percent sulphuric acid...
Excess of ammonia with sodium hypochloride solution in the presence of glue or gelatine gives (A) 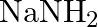 (B)
(B) 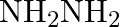 (C)
(C)  (D)
(D) 
Correct option is B $\mathrm{NH}_{2} \mathrm{NH}_{2}$ Explanation: Excess of ammonia with sodium hypo chloride solution in the presence of glue or gelatine gives hydrazine $\left(\mathrm{NH}_{2}...
Which among the following elements of group-2 exhibits anomalous properties ? (A) Be (B) Mg (C) Ca (D) Ba
A is the correct answer. Explanation: Be has anomalous characteristics. This is due to beryllium's smaller atomic and ionic radii when compared to the other members of the group. It's also because...
Phenol in presence of sodium hydroxide reacts with chloroform to form salicyladehyde. The reaction is known as (A) Kolbe’s reaction (B) ReimerTiermann reaction (C) Stephen reaction (D) Etard reaction
Correct option is B Explanation: Salicyladehyde is formed when phenol interacts with chloroform in the presence of sodium hydroxide. The Reimer-Tiemann reaction is the name of the reaction.
Bauxite, the ore of aluminium, is purified by which process ? (A) Hoope’s process (B) Hall’s process (C) Mond’s process (D) Liquation process
Correct option is B Explanation: (i)The Hall's process is the most widely used industrial method for smelting aluminium. The process entails dissolving aluminium oxide in molten cryolite and...
What are the products of auto-photolysis of water? (A) 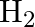 and
and 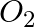 (B) Stream (C)
(B) Stream (C) 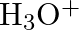 and
and 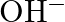 (D) Hydrogen peroxide
(D) Hydrogen peroxide
Correct Option is A Explanation: Water undergoes auto photolysis, which entails the breaking of chemical bonds as a result of the transmission of light energy to these bonds. It results in the...
In which among the following solids, Schottky defect is NOT observed ? (A) ZnS (B) NaCl (C) KCl (D) CsCl
Correct option is A (ZnS) Explanation: In ZnS, the Schottky defect does not exist. NaCl, KCl, and CsCl all have the Schottky defect. The Schottky defect occurs in solids where the cations and anions...
Two moles of an ideal gas are allowed to expand from a volume of 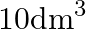 to
to 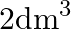 at
at 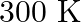 against a pressure of 101.325 KPa. Calculate the work done (A)
against a pressure of 101.325 KPa. Calculate the work done (A) 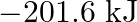 (B)
(B) 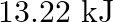 (C) -810.6kJ (D)
(C) -810.6kJ (D) 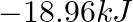
Correct option is A $-201.6 \mathrm{~kJ}$ Explanation: $ \begin{array}{l} \Delta \mathrm{V}=\mathrm{V}_{2}-\mathrm{V}_{1}=2 \mathrm{~m}^{3}-\left(10 \mathrm{dm}^{3} \times 10^{-3} \mathrm{~m}^{3} /...
