(1) – 900 kJ (2) + 270 kJ (3) – 900 J (4) + 900 kJ Correct Answer: (3) – 900 J Explanation: Using the formula, W = -Pext (Vf – Vi) W = -900 J
Test
The volume occupied by 1.8 g of water vapour at 374°C and 1 bar pressure will be [Use R = 0.083 bar LK–1 mol–1]
(1) 5.37 L (2) 96.66 L (3) 55.87 L (4) 3.10 L Correct Answer: (1) 5.37 L Explanation: Using the formula, PV = nRT V = 5.37 L
In hydrogen atom, the de Broglie wavelength of an electron in the second Bohr orbit is [Given that Bohr radius, ao = 52.9 pm]
(1) 105.8 pm (2) 211.6 pm (3) 211.6 pm (4) 52.9 pm Correct Answer: (3) 211.6 pm Explanation: n = 2 r = 211.6 2 pm
Orbital having 3 angular nodes and 3 total nodes is
(1) 6 d (2) 5 p (3) 3 d (4) 4 f Correct Answer: (4) 4 f Explanation: Total number of nodes = (n – 1) 3 = n – 1 n = 4
The density of 2 M aqueous solution of NaOH is 1.28 g/cm3. The molality of the solution is [Given that molecular mass of NaOH = 40 g mol-1]
(1) 1.32 m (2) 1.20 m (3) 1.56 m (4) 1.67 m Correct Answer: (4) 1.67 m Explanation: Let, volume of solution = 1 litre Mole of NaOH = 2 Mass of NaOH solution = 1000 × 1.28 = 1280 g Mass of...
The artificial sweetener stable at cooking temperature and does not provide calories is
(1) alitame (2) Saccharin (3) Aspartame (4) Sucralose Correct Answer: (4) Sucralose Explanation: Sucralose is trichloro derivative of sucrose. It is stable at cooking temperature. It does...
The polymer that is used as a substitute for wool in making commercial fibres is,
(1) Buna-N (2) Melamine (3) nylon-6,6 (4) Polyacrylonitrile Correct Answer: (4) Polyacrylonitrile Explanation: Polyacrylonitrile is used as a substitute for wool in making commercial fibre as...
Which structure of protein remain intact during denaturation process?
(1) Tertiary structure only (2) Both secondary and tertiary structures (3) Primary structure only (4) Secondary structure only Correct Answer: (3) Primary structure only Explanation: During...
The amine that reacts with Hinsberg’s reagent to give an alkali insoluble product is
Correct Answer: Option (2) Explanation: Secondary amines react with Hinsberg's reagent to give a product which is insoluble in...
The reaction that does not give benzoic acid as the major product is,
Correct Answer: Option (3) Explanation:
Which of the alkali metal chloride (MCl) forms its dihydrate salt (MCl . 2 H2O) easily?
(1) KCl (2) LiCl (3) CsCl (4) RbCl Correct Answer: (2) LiCl Explanation: Out of alkali metal chlorides only LiCl forms a dihydrate, other metal chlorides do not form hydrates.
Crude sodium chloride obtained by crystallization of brine solution does not contain
(1) CaSO4 (2) MgSO4 (3) Na2SO4 (4) MgCl2 Correct Answer: (2) MgSO4 Explanation: Crude sodium chloride generally obtained by crystallization of brine solution contains Na2SO4, CaSO4, CaCl2...
Which of the following is the correct order of dipole moment?
(1) H2O < NF3 < NH3 < BF3 (2) NH3 < BF3 < NF3 < H2O (3) BF3 < NF3 < NH3 < H2O (4) BF3 < NH3 < NF3 < H2O Correct Answer: (3) BF3 < NF3 < NH3 <...
Which of the following is paramagnetic?
(1) O2 (2) N2 (3) H2 (4) Li2 Correct Answer: (1) O2 Explanation: O2 has 8 + 8 = 16 electrons. In O2 molecule, there are two (2) unpaired electrons, so, it is a "paramagnetic" substance in...
The correct option representing a Freundlich adsorption isotherm is
(1) x/m = kp-1 (2) x/m = kp0.3 (3) x/m = kp2.5 (4) x/m = kp-0.5 Correct Answer: (2) x/m = kp0.3 Explanation: According to Freundlich isotherm, x/m = kp0.3
For a reaction, activation energy Ea = 0 and the rate constant at 200 K is 1.6 × 106 s-1. The rate constant at 400 K will be [Given that gas constant, R = 8.314 J K-1 mol-1]
(1) 3.2 × 106 s-1 (2) 3.2 × 104 s-1 (3) 1.6 × 106 s-1 (4) 1.6 × 103 s-1 Correct Answer: (3) 1.6 × 106 s-1 Explanation: From Arrhenius equation, k400 = k200
A first order reaction has a rate constant of 2.303 × 10-3 s-1. The time required for 40 g of this reactant to reduce to 10 g will be [Given that log102 = 0.3010]
(1) 602 s (2) 230.3 s (3) 301 s (4) 2000 s Correct Answer: (1) 602 s Explanation: For a first order reaction 40 g substance requires 2 half life periods to reduce upto 10 g Time taken in...
Following limiting molar conductivities are given as
$\lambda $mo (H2SO4) =x S cm2 mol-1 $\lambda $mo (K2SO4 )= y S cm2 mol-1 $\lambda $mo (CH3COOK) =z S cm mol-1 $\lambda $mo (in S cm2 mol-1 ) for CH3COOH will be (1)(x-y)/2+z (2) x – y + 2z (3) x +...
Which one is a wrong statement?
(1) The electronic configuration of N atom is, (2) An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers (3) Total orbital...
Iron exhibits bcc structure at room temperature. Above 900°C, it transforms to fcc structure. The ratio of density of iron at room temperature to that at 900°C (assuming molar mass and atomic radii of iron remains constant with temperature) is,
$\begin{array}{l} (1)\frac{{3\sqrt 3 }}{{4\sqrt 2 }}\\ (2)\frac{{4\sqrt 3 }}{{3\sqrt 2 }}\\ (3)\frac{{\sqrt 3 }}{{\sqrt 2 }}\\ (4)\frac{1}{2} \end{array}$ Correct Answer: (1)\frac{{3\sqrt 3...
Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is 1s2 2s2 2p3, the simplest formula for this compound is
(1) Mg2X (2) MgX2 (3) Mg2X3 (4) Mg3X2 Correct Answer: (4) Mg3X2 Explanation: Formula of compound formed by Mg and X will be Mg3X2.
Consider the following species: Which one of these will have the highest bond order?
(1) CN+ (2) CN- (3) NO (4) CN Correct Answer: (2) CN- Explanation: CN- = (s1s)2, (s* 1s)2, (s2s)2,(s* 2s)2, (p2px)2 = (p2py) 2,(s2pz) 2 Bond Order = 10-4 / 3 => 3
The correction factor ‘a’ to the ideal gas equation corresponds to
(1) Electric field present between the gas molecules (2) Volume of the gas molecules (3) Density of the gas molecules (4) Forces of attraction between the gas molecules Correct Answer: (4)...
The bond dissociation energies of X2, Y2 and XY are in the ratio of 1 : 0.5 : 1. H for the formation of XY is –200 kJ mol–1. The bond dissociation energy of X2 will be
(1) 800 kJ mol-1 (2) 100 kJ mol-1 (3) 200 kJ mol-1 (4) 400 kJ mol-1 Correct Answer: (1) 800 kJ mol-1 Explanation:
Following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations: a. 60 mL M /10 HCl + 40 mL M/10 NaOH b. 55 mL M/10 HCl + 45 mL M/10 NaOH c. 75 mL M/5 HCl + 25 mL M/5 NaOH d. 100 mL M/10 HCl + 100 mL M/10 NaOH pH of which one of them will be equal to 1?
(1) d (2) a (3) b (4) c Correct Answer: (4) c Explanation: \[\begin{array}{l} pH{\rm{ }} = {\rm{ }}--log\left[ {{H^ + }} \right]{\rm{ }}\\ {\rm{pH}} = {\rm{ - log}}\left[ {\frac{1}{{10}}}...
The geometry and magnetic behaviour of the complex [Ni(CO)4] are
(1) Square planar geometry and paramagnetic (2) Tetrahedral geometry and diamagnetic (3) Square planar geometry and diamagnetic (4) Tetrahedral geometry and paramagnetic Correct Answer: (2)...
The type of isomerism shown by the complex [CoCl2(en)2] is
(1) Ionization isomerism (2) Coordination isomerism (3) Geometrical isomerism (4) Linkage isomerism Correct Answer: (3) Geometrical isomerism Explanation:
Iron carbonyl, Fe(CO)5 is
(1) Trinuclear (2) Mononuclear (3) Tetranuclear (4) Dinuclear Correct Answer: (2) Mononuclear Explanation: Based on the number of metal atoms present in a complex, they are classified into...
Which one of the following ions exhibits d-d transition and paramagnetism as well?
(1) MnO4 - (2) Cr2O7 2- (3) CrO4 2- (4) MnO4 2- Correct Answer: (4) MnO4 2- Explanation: MnO4 2- - Unpaired electron (n) = 1; Paramagnetic
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II:
Column I Column II a. Co3+ i. 8 BM b. Cr3+ ii. 35 BM c. Fe3+ iii. 3 BM d. Ni2+ iv. 24 BM v. 15 BM Column I Column II a. Co3+ iv. 24 BM b. Cr3+ v. 15 BM c. Fe3+ ii. 35 BM d. Ni2+ i. 8...
Compound A, C8H10O, is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell. A and Y are respectively,
Correct Answer: Option (3) Explanation:
Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their,
(1) More extensive association of carboxylic acid via van der Waals force of attraction (2) Formation of carboxylate ion (3) Formation of intramolecular H-bonding (4) Formation of intermolecular...
In the reaction,
The electrophile involved is (1) Dichloromethyl anion CHCl2 (2) Formyl cation CHO (3) Dichloromethyl cation CHCl2 (4) Dichlorocarbene CCl2 Correct Answer: (4) Dichlorocarbene...
Which of the following is correct with respect to – I effect of the substituents? (R = alkyl)
(1) – NH2 > – OR > – F (2) – NR2 < – OR < – F (3) – NH2 < – OR < – F (4) – NR2 > – OR > – F Correct Answer: (1) – NH2 > – OR > – F (2) – NR2 < – OR < – F...
Which of the following carbocations is expected to be most stable?
Correct Answer: Option (1) Explanation: –NO2 group exhibit –I effect and it decreases with increase in distance. In option (1) positive charge present on...
Which of the following molecules represents the order of hybridisation sp2, sp2, sp, sp from left to right atoms?
(1) CH2 = CH – CH = CH2 (2) CH2 = CH – C º CH (3) HC º C – C º CH (4) CH3 – CH = CH – CH3 Correct Answer: (2) CH2 = CH – C º CH Explanation: Number of orbitals require in hybridization =...
Hydrocarbon (A) reacts with bromine by substitution to form an alkyl bromide which by Wurtz reaction is converted to gaseous hydrocarbon containing less than four carbon atoms. (A) is
(1) CH3– CH3 (2) CH2 = CH2 (3) CH º CH (4) CH4 Correct Answer: (4) CH4 Explanation:
The compound C7H8 undergoes the following reactions:
C_7H_8overset(3Cl_2//Delta)to A overset(Br_2//Fe)to B overset(Zn//HCl)to C
The product ‘C’ is
(1) 3-bromo-2,4,6-trichlorotoluene (2) o-bromotoluene (3) m-bromotoluene (4) p-bromotoluene Correct Answer: (1) 3-bromo-2,4,6-trichlorotoluene Explanation:
The compound A on treatment with Na gives B, and with PCl5 gives C. B and C react together to give diethyl ether. A, B and C are in the order
1) C2H5Cl, C2H6, C2H5OH (2) C2H5OH, C2H5Cl, C2H5ONa (3) C2H5OH, C2H6, C2H5Cl (4) C2H5OH, C2H5ONa, C2H5Cl Correct Answer: (4) C2H5OH, C2H5ONa, C2H5Cl Explanation:
Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity?
(1) N2O (2) NO2 (3) N2O5 (4) NO Correct Answer: (3) N2O5 Explanation: N2O5 is the oxide of nitrogen which is not a common pollutant introduced into the atmosphere both due to natural and...
Which of the following oxides is most acidic in nature?
(1) BaO (2) BeO (3) MgO (4) CaO Correct Answer: (2) BeO Explanation: BeO is amphoteric oxide while other given oxides are basic.
A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with conc. H2SO4. The evolved gaseous mixture is passed through KOH pellets. Weight (in g) of the remaining product at STP will be
(1) 2.8 (2) 3.0 (3) 1.4 (4) 4.4 Correct Answer: (1) 2.8 Explanation: Gaseous mixture formed is CO and CO2 when it is passed through KOH, only CO2 is absorbed. So...
The difference between amylose and amylopectin is
(1) Amylopectin have 1 $ \to $ 4 a-linkage and 1 $ \to $ 6 b-linkage (2) Amylose have 1 $ \to $ 4 a-linkage and 1 $ \to $ 6 b-linkage (3) Amylopectin have 1 $ \to $ 4 a-linkage and 1 $ \to $ 6...
Nitration of aniline in strong acidic medium also gives m-nitroaniline because
(1) In absence of substituents nitro group always goes to m-position. (2) In electrophilic substitution reactions amino group is meta directive. (3) Inspite of substituents nitro group always goes...
When initial concentration of the reactant is doubled, the half-life period of a zero-order reaction
(1) Is tripled (2) Is doubled (3) Is halved (4) Remains unchanged Correct Answer: (2) Is doubled Explanation: When initial concentration of the reactant is doubled, the half-life period of a...
Which one of the following conditions will favour maximum formation of the product in the reaction,
A_(2) (g) + B_(2) (g) hArr X_(2) (g) , Delta_(r) H= -x kJ?
(1) High temperature and high pressure (2) Low temperature and low pressure (3) Low temperature and high pressure (4) High temperature and low pressure Correct Answer: (3) Low temperature and...
For the redox reaction, MnO4- + C2O42- + H+ → Mn2+ + CO2 + H2O The correct coefficients of the reactants for the balanced equation are,
MnO4- C2O42- H+ (1) 2 16 5 (2) 2 5 16 (3) 16 ...
Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below:
Then the species undergoing disproportionation is, (1) Br2 (2) BrO4- (3) BrO3- (4) HBrO Correct Answer: (4) HBrO Explanation: $\begin{array}{l} {E^o}_{cell} =...
Among CaH2, BeH2, BaH2, the order of ionic character is
(1) BeH2 < BaH2 < CaH2 (2) CaH2 < BeH2 < BaH2 (3) BeH2 < CaH2 < BaH2 (4) BaH2 < BeH2 < CaH2 Correct Answer: (3) BeH2 < CaH2 < BaH2 Explanation: On moving down...
The correct difference between first and second order reactions is that
(1) A first-order reaction can catalyzed; a second-order reaction cannot be catalyzed (2) The half-life of a first-order reaction does not depend on [A]0; the half-life of a second-order reaction...
In which case is number of molecules of water maximum?
(1) 0.00224 L of water vapours at 1 atm and 273 K (2) 0.18 g of water (3) 18 mL of water (4) 10–3 mol of water Correct Answer: (3) 18 mL of water Explanation: In 18ml of water, the number of...
The solubility of BaSO4 in water is 2.42 × 10–3 gL–1 at 298 K. The value of its solubility product (Ksp) will be (Given molar mass of BaSO4 = 233 g mol–1)
(1) 1.08 × 10-14 mol2L-2 (2) 1.08 × 10-12 mol2L-2 (3) 1.08 × 10-10 mol2L-2 (4) 1.08 × 10-8 mol2L-2 Correct Answer: (3) 1.08 × 10-10 mol2L-2 Explanation: $\begin{array}{l} {K_{sp}} = [B{a^{2 +...
Given van der Waals constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied?
(1) O2 (2) H2 (3) NH3 (4) CO2 Correct Answer: (3) NH3 Explanation: The van der Waal constant ‘a’, signifies intermolecular forces of attraction. As higher is the value of ‘a’, easier will be...
On which of the following properties does the coagulating power of an ion depend?
(1) Both magnitude and sign of the charge on the ion (2) Size of the ion alone (3) The magnitude of the charge on the ion alone (4) The sign of charge on the ion alone. Correct Answer: (1)...
Regarding cross-linked or network polymers, which of the following statements is incorrect?
(1) Examples are bakelite and melamine. (2) They are formed from bi- and tri-functional monomers. (3) They contain covalent bonds between various linear polymer chains. (4) They contain strong...
Which of the following compounds can form a zwitterion?
(1) Benzoic acid (2) Acetanilide (3) Aniline (4) Glycine Correct Answer: (4) Glycine Explanation:
Identify the major products P, Q and R in the following sequence of reactions:
Correct Answer: Option (4) Explanation:
In the structure of ClF3, the number of lone pair of electrons on central atom ‘Cl’ is
(1) Four (2) Two (3) One (4) Three Correct Answer: (2) Two Explanation:
Which of the following statements is not true for halogens?
(1) All but fluorine shows positive oxidation states (2) All are oxidizing agents (3) All form monobasic oxyacids (4) Chlorine has the highest electron-gain enthalpy Correct Answer: (1) All...
The correct order of atomic radii in group 13 elements is
(1) B < Ga < Al < Tl < In (2) B < Al < Ga < In < Tl (3) B < Al < In < Ga < Tl (4) B < Ga < Al < In < Tl Correct Answer: (4) B < Ga < Al...
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
(1) Mg (2) Zn (3) Fe (4) Cu Correct Answer: (1) Mg Explanation: Metal that is more reactive than aluminium can reduce alumina so that Magnesium can reduce alumina.
Which one of the following elements is unable to form MF6 3– ion?
(1) B (2) Al (3) Ga (4) In Correct Answer: (1) B Explanation: The element boron has no vacant d-orbitals in its valence shell as it cannot extend its covalency more than 4.
The correct order of N-compounds in its decreasing order of oxidation states is
(1) HNO3, NH4Cl, NO, N2 (2) HNO3, NO, NH4Cl, N2 (3) HNO3, NO, N2, NH4Cl (4) NH4Cl, N2, NO, HNO3 Correct Answer: (3) HNO3, NO, N2, NH4Cl Explanation: The correct order of N-compounds in its...
In the extraction of copper from its sulphide ore, the metal is finally obtained by the reduction of cuprous oxide with:
(1) Copper (I) sulphide (2) Sulphur dioxide (3) Iron (II) sulphide (4) Carbon monoxide Correct Answer: (2) Sulphur dioxide Explanation: Reaction Involved – Cu2S + 2Cu2O ⟶ 6Cu + SO2 ↑ ...
Which is the correct order of increasing energy of the listed orbitals in the atom of titanium? (At. No. Z = 22)
(1) 3s 3p 3d 4s (2) 3s 3p 4s 3d (3) 3s 4s 3p 3d (4) 4s 3s 3p 3d Correct Answer: (2) 3s 3p 4s 3d Explanation: Ti(22) = 1s22s22p63s23p64s23d2
Which of the following reaction(s) can be used for the preparation of alkyl halides?
(I) CH3CH2OH + HCl → (anhydrous ZnCl2) (II) CH3CH2OH + HCl → (III) (CH3)3COH + HCl → (IV) (CH3)2CHOH + HCl → (anhydrous ZnCl2) (1) IV only (2) III and IV only (3) I, III and IV only (4) I...
Method by which Aniline cannot be prepared is:
(1) Reduction of nitrobenzene with H2⁄Pd in ethanol. (2) Potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution. (3) Hydrolysis of...
Which of the following is not the product of dehydration of
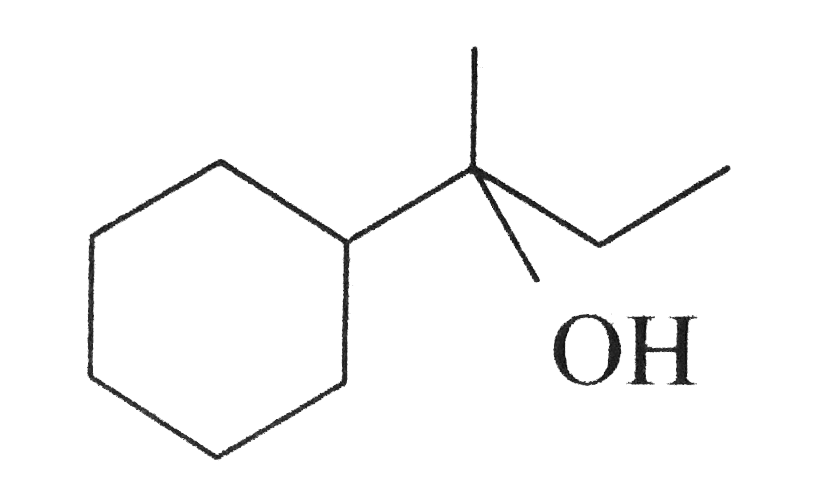 ?
?
Correct Answer: Option (d) Explanation:
The oxidation of benzene by V2O5 in the presence of air produces:
(1) Benzoic acid (2) Benzaldehyde (3) Benzoic anhydride (4) Maleic anhydride Correct Answer: (4) Maleic anhydride Explanation:
What is the mass of the precipitate formed when 50 mL of 16.9% solution of AgNO3 is mixed with 50 mL of 5.8% NaCl solution?
(1) 7 g (2) 14 g (3) 28 g (4) 3.5 g Correct Answer: (4) There is strong hydrogen bonding between HF molecules. Explanation: Calculation - Mass of AgCl precipitated = 0.049 × 143.5 g => 7 g...
The variation of the boiling points of the hydrogen halides is in the order HF > HI > HBr > HCl. What explains the higher boiling point of hydrogen fluoride?
(1) The bond energy of HF molecules is greater than in other hydrogen halides. (2) The effect of nuclear shielding is much reduced in fluorine which polarizes the HF molecule. (3) The...
The rate constant of the reaction A ⟶ B is 0.6 × 10−3 mole per second. If the concentration of A is 5M, then concentration of B after 20 minutes is:
(1) 0.36 M (2) 0.72 M (3) 1.08 M (4) 3.60 M Correct Answer: (2) 0.72 M Explanation: Calculation - x = K x t x = 0.6 × 10−3 × 20 × 60 x = 0.72 M
In an SN1 reaction on chiral centres, there is:
(1) 100% retention (2) 100% inversion (3) 100% racemization (4) Inversion more than retention leading to partial racemization Correct Answer: (4) Inversion more than retention leading to...
Which one of the following esters gets hydrolyzed most easily under alkaline conditions?
(1) (2) (3) (4) Correct Answer: (4) Hydrazine in presence of feebly acidic solution Explanation: The electron withdrawing group attach to the benzene ring increases the reactivity towards...
Reaction of a carbonyl compound with one of the following reagents involves nucleophilic addition followed by elimination of water. The reagent is:
(A) Hydrocyanic acid (B) Sodium hydrogen sulphite (C) A Grignard reagent (D) Hydrazine in presence of feebly acidic solution Correct Answer: (D) Hydrazine in presence of feebly acidic...
The sum of coordination number and oxidation number of metal M in the complex [M(en)2 (C2O4 )]Cl (Where en is ethylenediamine) is:
(1) 7 (2) 8 (3) 9 (4) 6 Correct Answer: (3) 9 Explanation: Sum of oxidation state + Coordination number = 3 + 6 = 9
The following reaction is known by the name:
(1) Acetylation reaction (2) Schotten-Baumen reaction (3) Friedel-Craft’s reaction (4) Perkin’s reaction Correct Answer: (2) Schotten-Baumen reaction Explanation: The name of...
Two possible stereo-structures of CH3CHOH ∙ COOH, which are optically active, are called:
(1) Enantiomers (2) Mesomers (3) Diastereomers (4) Atropisomers Correct Answer: (1) Enantiomers Explanation:
The stability of +1 oxidation state among Al, Ga, In and TI increases in the sequence:
(1) TI < In < Ga < Al (2) In < TI < Ga < Al (3) Ga < In < Al < TI (4) Al < Ga < In < TI Correct Answer: (4) Al < Ga < In < TI Explanation: The...
The correct statement regarding defects in crystalline solids is :
(1) Frenkel defect is a dislocation defect (2) Frenkel defect is found in halides of alkaline metals (3) Schottky defects have no effect on the density of crystalline solids (4) Frenkel defects...
What is the mole fraction of the solute in a 1.00 m aqueous solution?
(1) 0.0354 (2) 0.0177 (3) 0.177 (4) 1.770 Correct Answer: (2) 0.0177 Explanation: XSolute = nSolute / nSolute + nH2O
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample? (Atomic weight: Mg = 24)
(1) 60 (2) 84 (3) 75 (4) 96 Correct Answer: (2) 84 Explanation: Reaction Involved – MgCO3(s) ⟶ MgO(s) + CO2(g) 0.238 mole MgCO3 will give 0.238 mole MgO = 0.238 × 40 g = 9.523g MgO Practical...
The heat of combustion of carbon to CO2 is -393.5 kJ/mol. The heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas is:
(1) −630 kJ (2) −3.15 kJ (3) −315 kJ (4) +315 kJ Correct Answer: (3) −315 kJ Explanation: Reaction Involved - C(s) + O2(g) → CO2(g) ; ∆fH = −393.5 kJ mol −1 Heat released upon formation of...
The hybridization involved in complex [Ni(CN)4 ] 2− is: (Atomic number of Ni = 28)
(1) d2 sp2 (2) d2 sp3 (3) dsp2 (4) sp3 Correct Answer: (3) dsp2 Explanation: Calculation – The oxidation state of Ni = +2 x − 4 = 2 x = +2
Which of the following statements is not correct for a nucleophile?
(1) Nucleophiles attack low e − density sites (2) Nucleophiles are not electron seeking (3) Nucleophile is a Lewis acid (4) Ammonia is a nucleophile Correct Answer: (3) Nucleophile is a Lewis...
If Avogadro number NA , is changed from 6.022 × 1023 mol −1 to 6.022 × 1020 mol −1, this would change:
(1) The ratio of chemical species to each other in a balanced equation. (2) The ratio of elements to each other in a compound. (3) The definition of mass in units of grams (4) The mass of one mole...
The name of complex ion, [Fe(CN)6]3− is :
(1) Tricyanoferrate (III) ion (2) Hexacyanidoferrate (III) ion (3) Hexacyanoiron (III) ion (4) Hexacyanitoferrate (III) ion Correct Answer: (2) Hexacyanidoferrate (III) ion Explanation:...
Which of the statements given below is incorrect?
(1) ONF is isoelectronic with O2N – (2) OF2 is an oxide of fluorine (3) Cl2O7 is an anhydride of perchloric acid (4) O3 molecule is bent Correct Answer: (2) OF2 is an oxide of fluorine...
The vacant space in bcc lattice unit cell is:
(1) 23% (2) 32% (3) 26% (4) 48% Correct Answer: (2) 32% Explanation: The vacant space in bcc lattice = 100 − 68 The vacant space in bcc lattice = 32%
Reaction of phenol with chloroform in presence of dilute sodium hydroxide finally introduces which one of the following functional group?
(1) −CHCl2 (2) – CHO (3) – CH2Cl (4) – COOH Correct Answer: (2) – CHO Explanation: The reaction of phenol and chloroform in the presence of dilute sodium hydroxide ultimately introduces the...
Assuming complete ionization, same moles of which of the following compounds will require the least amount of acidified KMnO4 for complete oxidation?
(1) FeC2O4 (2) Fe(NO2)2 (3) FeSO4 (4) FeSO3 Correct Answer: (3) FeSO4 Explanation: Oxidation of 1 mole of FeC2O4 requires 3/5 moles of acidified KMnO4. Oxidation of 1 mole of Fe(NO2)2...
In the reaction with HCl, an alkene reacts in accordance with the Markovnikov’s rule, to give a product 1-chloro-1- methylcyclohexane. The possible reaction alkene is:
Correct Answer: Option (c )
In which of the following pairs, both the species are not isostructural?
(1) NH3, PH3 (2) XeF4, XeO4 (3) SiCl4, PCl4+ (4) Diamond, silicon carbide Correct Answer: (2) XeF4, XeO4 Explanation: Structure of NH3 and PH3 is tetrahedral. Structure of XeF4...
The number of water molecules is maximum in:
(1) 18 gram of water (2) 18 moles of water (3) 18 molecules of water (4) 1.8 gram of water Correct Answer: (2) 18 moles of water Explanation: Water molecules present in 18 mole of water = 18...
Decreasing order of stability of O2, O2−, O2+ and O2 2− is:
(1) O2 > O2+ > O22- > O2- (2) O2- > O22-> O2+ > O2 (3) O2+ > O2 > O2- > O22- (4) O22- > O2- > O2 > O2+ Correct Answer: (3) O2+ > O2 > O2- > O22-...
Strong reducing behaviour of H3PO2 is due to:
(1) High oxidation state of phosphorus (2) Presence of two –OH groups and one p – H bond (3) Presence of one –OH group and two p – H bonds (4) High electron gain enthalpy of phosphorus ...
On heating which of the following releases CO2 most easily?
(1) MgCO3 (2) CaCO3 (3) K2CO3 (4) Na2CO3 Correct Answer: (1) MgCO3 Explanation: Thermal stability order - K2CO3 > Na2CO3 > CaCO3 > MgCO3 So, MgCO3 releases CO2 most easily. Reaction...
Caprolactum is used for the manufacture of:
(1) Terylene (2) Nylon-6,6 (3) Nylon-6 (4) Teflon Correct Answer: (3) Nylon-6 Explanation:
Aqueous solution of which of the following compounds is the best conductor of electric current?
(1) Ammonia, NH3 (2) Fructose, C6H12O6 (3) Acetic acid, C2H4O2 (4) Hydrochloric acid, HCl Correct Answer: (4) Hydrochloric acid, HCl Explanation: The aqueous solution of Hydrochloric acid is...
Which one of the following pairs of solution is not an acidic buffer?
(1) H2CO3 and Na2CO3 (2) H3PO4 and Na3PO4 (3) HClO4 and NaClO4 (4) CH3COOH and CH3COONa Correct Answer: (3) HClO4 and NaClO4 Explanation: HClO4 and NaClO4 cannot form a buffer solution....
Which of the following complexes is used to be as an anticancer agent? (Option A mer-[Co (NH3)3Cl3])( Option B cis-[PtCl2 (NH3)2])( Option C cis-K2 [PtCl2Br2] )(Option D Na2CoCl4)
Correct Answer: (Option B cis-[PtCl2 (NH3)2]) Explanation: Cis-platin is used as an anticancer agent
Reason of lanthanoid contraction is:– (Option A – Negligible screening effect of ‘f’ orbitals)( Option B – Increasing nuclear charge) (Option C – Decreasing nuclear charge) (Option D – Decreasing screening effect)
Correct Answer: (Option A Negligible screening effect of 'f' orbitals) Explanation: Due to poor shielding of f-orbitals, nucleus will exert a strong attraction, causes lanthanoid contraction
In the following reaction, the product (A)
Correct Answer: (Option D) Explanation:
Which of the following will be most stable diazonium salt RN2+X-? (Option A -CH3N2+X-) (Option B – C6H5N2+X-) (Option C – CH3CH2N2+X-) (Option D – C6H5 CH2N2+X-)
Correct Answer: (Option B) Explanation: Primary aliphatic amines form highly unstable alkyl diazonium salts. Primary aromatic amines form arene diazonium salts which are stable for a short time in...
D (+) glucose reacts with hydroxylamine and yields an oxime. The structure of the oxime would be
Correct Answer: (Option D) Explanation:
Which of the following hormones is produced under the condition of stress which stimulates glycogenolysis in the liver of human beings? (Option A Thyroxin)( Option B Insulin)( Option C Adrenaline) (Option D Estradiol)
Correct Answer: (Option C Adrenaline) Explanation: Adrenaline is produced under the condition of stress which stimulates glycogenolysis in the liver of human beings
Which one of the following is an example of a thermosetting polymer?
Correct Answer: (Option D) Explanation: Bakelite is a thermosetting polymer. Thermosetting polymers are cross linked or heavily branched...
Which of the following organic compounds polymerizes to form the polyester Dacron?( Option A Propylene and para HO – (C6H4) – OH)( Option B Benzoic acid and ethanol)( Option C Terephthalic acid and ethylene glycol) (Option D Benzoic acid and para HO – (C6H4) – OH)
Correct Answer: (Option C Terephthalic acid and ethylene glycol) Explanation: Dacron or terylene is the best known example of polyesters. It is manufactured by heating a mixture of ethylene glycol...
Which one of the following is not a common component of Photochemical Smog?( Option A Ozone) (Option B Acrolein)( Option C Peroxyacetyl nitrate) (Option D Chlorofluorocarbons)
Correct Answer: (Option D Chlorofluorocarbons) Explanation: The common components of photochemical smog are ozone, nitric oxide, acrolein, formaldehyde and peroxyacetyl nitrate (PAN). Hence...
In the Kjeldahl’s method for estimation of nitrogen present in a soil sample, ammonia evolved from 0.75 gm of sample neutralized 10 mL of 1 M H2SO4, The percentage of nitrogen in the soil is;( Option A 37.33 )(Option B 45.33)( Option C 35.33)( Option D 43.33)
Correct Answer: (Option A 37.33 ) Explanation: By using the Kjeldahl’s method, %N = 37.33%
What products are formed when the following compound is treated with Br2 in the presence of FeBr3?
Correct Answer: (Option D) Explanation:
Which of the following compounds will undergo racemisation when solution of KOH hydrolyses?
Correct Answer: Option (ii) Explanation:
Among the following sets of reactants which one produces anisole?( Option A CH3CHO; RMgX) (Option B C6H5OH; NaOH; CH3l)( Option C C6H5OH; neutral FeCl3 )(Option D C6H5 – CH3; CH3COCl; AlCl3)
Correct Answer: (Option D) Explanation:
Which of the following will not be soluble in sodium hydrogen carbonate? (Option A 2, 4, 6-trinitrophenol) (Option B Benzoic acid) (Option C o-Nitrophenol) (Option D Benzenesulphonic acid)
Correct Answer: (Option C o-Nitrophenol) Explanation:
Which one is most reactive towards Nucleophilic addition reaction?
Correct Answer: (Option 4) Explanation:
Identify Z in the sequence of reactions:
CH_(3)CH_(2)CH=CH_(2)underset(H_(2)O_(2))overset(HBr)rarrYoverset(C_(2)H_(6)ONa)rarrZ (Option – CH3-(CH2)3-OCH2CH3) (Option B – (CH3)2CH2-O-CH2CH3) (Option C – CH3(CH2)4-O-CH3) (Option D – CH3CH2-CH(CH3)-O-CH2CH3)
Correct Answer: Option A Explanation:
Which of the following organic compounds has same hybridization as its combustion product CO2? (Option A Ethane) (Option B Ethyne) (Option C Ethene) (Option D Ethanol)
Correct Answer: Option B Explanation:
Be2+ is isoelectronic with which of the following ions? (Option A H+ )(Option B Li+ )(Option C Na+ )(Option D Mg2+ )
Correct Answer: (Option B Li+ ) Explanation: Li+, Be+2 & Li+ have 2 electron
Which of the following molecules has the maximum dipole moment? (Option A CO2 )(Option B CH4 )(Option C NH3 )(Option D NF3)
Correct Answer: (Option B CH4 ) Explanation:
Which one of the following species has plane triangular shape? (Option A N3 )(Option B 3 NO3- )(Option C 2 NO2- Option D CO2)
Correct Answer: (Option B 3 NO3- ) Explanation: NO3- has Sp2 hybridisation i.e. has planar shape.
Acidity of diprotic acids in aqueous solutions increases in the order:– (Option A H2S < H2Se < H2 Te) (Option B H2Se < H2S < H2Te )(Option C H2Te < H2S < H2Se)( Option D H2 Se < H2Te < H2S)
Correct Answer: (Option A H2S < H2Se < H2 Te) Explanation: On moving down the group bond length increases, and bond strength decreases so release of H becomes easier and acidic strength...
(a) H2O2 +O3 → H2 O +2O2 (b)H2O2 + Ag2 O → 2Ag + H2O + O2 Role of hydrogen peroxide in the above reactions is respectively- (Option A Oxidizing in (a) and reducing in)( (b) Option B Reducing in (a) and oxidizing in (b))( Option C Reducing in (a) and (b))( Option D Oxidizing in (a) and (b))
Correct Answer: (Option C Reducing in (a) and (b)) Explanation: Hydrogen peroxide acts as reducing agent in presence of strong oxidizing agents KMnO4, K2Cr2 O7, ozone etc
Artificial sweetener which is stable under cold conditions only is:– (Option A Saccharine)( Option B Sucralose )(Option C Aspartame)( Option D Alitame)
Correct Answer: (Option C Aspartame) Explanation: Aspartame artificial sweetner which is stable under cold conditions only.
In acidic medium, H2O2 changes Cr2O7 -2 to CrO5 which has two (–O–O) bonds. Oxidation state of Cr in CrO5 is:– (Option A +5)( Option B +3)( Option C +6)( Option D -10)
Correct Answer: ( Option C - +6) Explanation: Oxidation state of Cr in CrO5 is +6.
The reaction of aqueous KMnO4 with H2O2 in acidic conditions gives:–( Option A Mn4+ and O2 )(Option B Mn2+ and O2 )(Option C Mn2+ and O3 )(Option D Mn4+ and MnO2 )
Correct Answer: (Option B Mn2+ and O2 ) Explanation: KMnO4 is a strong oxidlsing agent & will oxidise H2O2 to O2. KMnO4 H2 O2 Mn+2 O2
Among the following complexes the one which shows zero crystal field stabilization energy (CFSE) is:– (Option A [Mn (H2O) 6]3+ )(Option B [Fe (H2O) 6]3+ )(Option C [Co (H2O) 6]2+ )(Option D [Co (H2O) 6]3+)
Correct Answer: (Option B [Fe (H2O) 6]3+ ) Explanation: Due to d5 configuration and H2O is a weak ligand Fe3+: t 32g e2g
Magnetic moment 2.83 BM is given by which of the following ions? (At. nos. Ti = 22, Cr = 24, Mn = 25, Ni = 28): (Option A Ti3+ )(Option B Ni2+ )(Option C Cr3+ )(Option D Mn2+)
Correct Answer: (Option B Ni2+) Explanation: Ni+2 has two unpaired electron. Ni+2 : [Ar]3d8 configuration
When 0.1 mol MnO42– is oxidised the quantity of electricity required to completely oxidise MnO42- to MnO4- is: – (Option A – 96500 C) (Option B – 2 × 96500 C) (Option C – 9650 C) (Option D -96.50 C)
Correct Answer: (Option C - 9650 C) Explanation: In the given reaction. Charge required = 0.1 F => 0.1 69500 => 9650C
Using the Gibbs energy change, ΔG° = +63.3 kJ, for the following reaction, Ag2CO3 → 2Ag+(aq) +CO32- (aq) The Ksp of Ag2CO3 (s) in water at 25°C is: – (R=8.314 J K-1 mol-1) (Option A – 3 .2 × 10-26) (Option B – 8 .0 × 10-12) (Option C – 2.9 × 10-3) (Option D – 7.9 × 10-2)
Correct Answer: (Option B - 8 .0 × 10-12) Explanation: ΔG° = -2.303 RT log Ksp 63.3 × 1000 = -2.303 × 8.314 × 298 log Ksp log Ksp = -11.09 Ksp = Antilog(-11.09) = 8 × 10-12
The weight of silver (at wt. = 108) displaced by a quantity of electricity which displaces 5600 mL of O2 at STP will be:– (Option A 5.4 g) (Option B 10.8 g) ( Option C 54.0 g) ( Option D 108.0 g )
Correct Answer: (Option D 108.0 g) Explanation: According to Faraday's 2nd law, $\begin{array}{l} \frac{{{w_{AG}}}}{{{E_{AG}}}} = \frac{{{w_{O2}}}}{{{E_{O2}}}}\\ \frac{{{w_{AG}}}}{{108}} =...
Which of the following statements is correct for the spontaneous adsorption of a gas?( Option A ΔS is negative and, therefore, ΔH should be highly positive) (Option B ΔS is negative and therefore, ΔH should be highly negative) (Option C ΔS is positive and, therefore, ΔH should be negative) (Option D ΔS is positive and, therefore, ΔH should also be highly positive)
Correct Answer: (Option B ΔS is negative and therefore, ΔH should be highly negative) Explanation: During adsorption entropy decreases, so ΔS < 0 ΔG = ΔH – TΔS For spontaneous adsorption ΔG <...
For the reversible reaction: N2 (g) + 3H2 (g) ⇌ 2N3 (g) + Heat The equilibrium shifts in forward direction:( Option A By Increasing the concentration of NH2 (g)) (Option B By decreasing the pressure )(Option C By decreasing the concentrations of N2 (g) and H2 (g)) (Option D By increasing pressure and decreasing temperature)
Correct Answer: (Option D By increasing pressure and decreasing temperature) Explanation: According to Le-Chatelier's Principle → In exothermic reactions low temperature favours the forward...
For the reaction: X2O4 (ℓ) → 2 XO2 (g) ΔU = 2.1 k cal, ΔS = 20 cal K-1 at 300 K Hence ΔG is:-( Option A 2.7 k cal) (Option B –2.7 k cal) (Option C 9.3 k cal) (Option D –9.3 k cal)
Correct Answer: (Option B –2.7 k cal) Explanation: X2O4 (ℓ) → 2 XO2 (g); Δng = 2 – 0 = 2 ΔH = ΔU + ΔngRT $ = 2.1 + 2 \times \frac{2}{{1000}} \times 300$ ΔH = 3.3 kcal ΔG = ΔH – T.ΔS $ = 3.3 - 300...
For a given exothermic reaction Kp and K’p are the equilibrium constant at temperatures T1 and T2, respectively. Assuming that heat of reaction is constant in temperature range between T1 and T2, it is readily observed that:- (Option A Kp > K’p )(Option B Kp < K’p )(Option C Kp = K9')
Correct Answer: (Option A Kp > K’p ) Explanation: In exothermic reactions on increasing temperature value of Kp decreases. So, Kp > Kp'
Which of the following orders of ionic radii is correctly represented? (Option A H_ > H > H+ )( Option B Na+ > F_ > O2–)( Option C F- > O2- > Na+ )(Option D Al3+ > Mg2+ > N3- )
Correct Answer: (Option A H_ > H > H+ ) Explanation: H has one electron and one proton. If one more electron is added to the shell, the effective nuclear charge per electron decreases and...
1.0 g of magnesium is burnt with 0.56 g O2 in a closed vessel. Which reactant is left in excess and how much? (At. wt. Mg = 24; O = 16) (Option A Mg, 0.16 g)( Option B O2, 0.16 g )(Option C Mg, 0.44 g )(Option D O2, 0.28 g)
Correct Answer: (Option A Mg, 0.16 g) Explanation: Calculation - Mass of Mg = 0.0066 × 24g = 0.16 g
The pair of compounds that can exist together is: (Option A FeCl3, SnCl2 )(Option B HgCl2, SnCl2 )(Option C FeCl2, SnCl2 )(Option D FeCl2. KI)
Correct Answer: (Option C FeCl2, SnCl2 ) Explanation: FeCl2, SnCl2 are reducing agents
When 22.4 litres of H2 (g) is mixed with 11.2 litres of Cl2 (g), each at S.T. P., the moles of HCl (g) formed is equal to: (Option A – 1 mol of HCl (g)) (Option B – 2 mol of HCl (g)) (Option C – 0 .5 mol of HCl) (Option D – 1.5 mol of HCl (g))
Correct Answer: (Option A - 1 mol of HCl (g)) Explanation:
When 22.4 litres of H2 (g) is mixed with 11.2 litres of Cl2 (g), each at S.T. P., the moles of HCl (g) formed is equal to: (Option A – KCI) (Option B – C6H12O6) (Option C – Al2 (SO4)3) (Option D – K2SO4)
Correct Answer: (Option C – Al2 (SO4)3) Explanation: Depression in freezing point ∝ van’t Hoff's factor (i) for Al2 (SO4)3, i = 5
Which of the following salts will give highest pH in water? (Option A – KCl) (Option B – NaCl) (Option C – Na2CO3) (Option D – CuSO4)
Correct Answer: (Option C – Na2CO3) Explanation: Salt of strong base and weak acid will give highest pH in water.
Which property of colloids is not dependent on the charge on colloidal particles? (Option A – Coagulation) (Option B – Electrophoresis) (Option C – Electro–osmosis) (Option D – Tyndall effect)
Correct Answer: (Option D - Tyndall effect) Explanation: Tyndall effect is optical property. It is not dependent on the charge on colloidal particles.
If a is the length of the side of a cube, the distance between the body centered atom and one corner atom in the cube will be: Options – (Option A – 2/√3a) (Option B – 4/√3a) (Option C – √3/4a) (Option D – √3/2a)
Correct Answer: (Option D) Explanation: The distance between the body centered atom and one corner atom is a/2.
Equal masses of H2, O2 and methane have been taken in a container of volume V at temperature 27°C in identical conditions. The ratio of the volumes of gases H2: O2: methane would be: (Option A – 8: 16: 1) (Option B – 16: 8: 1) (Option C – 16: 1: 2) (Option D – 8: 1: 2)
Correct Answer: (Option C - 16: 1: 2) Explanation: volume ∝ moles nH2 = w/2 no2 = w/32 nCH4 = w/16 Ratio = 16:1:2
Calculate the energy in joule corresponding to light of wavelength 45 nm: (planck’s constant h = 6.63 × 10-34 Js; speed of light c = 3 × 108 ms-1) (Option A – 6.67 × 1015) (Option B – 6.67 × 1011) (Option C – 4 .42 × 10-15) (Option D – 4.42 × 10-18)
Correct Answer: (Option D - 4.42 × 10-18) Explanation: Energy = hc/l $E = \frac{{6.63 \times {{10}^{ - 34}} \times 3 \times {{10}^8}}}{{45 \times {{10}^{ - 9}}}}$ E = 4.42 × 10-18J
What is the maximum number of orbitals that can be identified with the following quantum numbers? n = 3, ℓ = 1, mℓ = 0. (Option A – 1) (Option B – 2) (Option C – 3) (Option D – 4)
Correct Answer: (Option A - 1) Explanation: n = 3, ℓ = 1, m = 0 Orbital is 3pz So only one orbital can be identified with these quantum numbers.
Which is the strongest acid in the following?
Option A H2SO3 Option B H2SO4 Option C HCIO3 Option D HCIO4 Solution: Correct Option D The higher the oxidation number of the central atom more the acidity. So, the oxidation number for all the...
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 20°C to 35°C? (R = 8.314 J/mol-K)
Option A 15.1 k J/mol Option B 342 k J/mol Option C 269 k J/mol Option D 34.7 k J/mol Solution: Correct Option D
Identify the correct order of solubility in aqueous medium:
Option A Na2 S> ZnS > CuS Option B CuS > ZnS > Na2S Option C ZnS > Na2 S > CuS Option D Na2S < CuS > ZnS Solution: Correct Option A We will make use of the fact that the...
Which of the following is paramagnetic?
Option A NO+ Option B CO Option C O2⎺ Option D CN⎺ Solution: Correct Option C O2- → 15 e- contains one unpaired electron in Anti bonding molecular orbital (ABMO).
Maximum deviation from ideal gas is expected from:
Option A NH3(g) Option B H2(g) Option C N2(g) Option D CH4(g) Solution: Correct Option A NH3 will show maximum deviation from ideal gas because of the dipole-dipole attraction.
 molecule of urea are present in 100mL of its solution. The concentration of solution is:-
molecule of urea are present in 100mL of its solution. The concentration of solution is:-
Option A 0.1 M Option B 0.02 M Option C 0.01 M Option D 0.001 M Solution: Correct Option C The number of moles of urea to (volume in L) of the solution equals the concentration of the solution. The...
Which is the monomer of Neoprene in the following:-
Which of the following compounds will not undergo Friedal-Craft’s reaction easily:-
Option A Toluene Option B Cumene Option C Xylene Option D Nitrobenzene Solution: Correct Option D When a strong deactivating group, such as nitro, is added to the benzene ring, the Fridel Craft...
Which of these is least likely to act as a Lewis base?
Option A PF3 Option B CO Option C F[1] Option D BF3 Solution: Correct Option D BF3 is Lewis acid (e− pair acceptor)
A button cell used in watches functions as following:
Option A 1.34 V Option B 1.10 V Option C 0.42 V Option D 0.84 V Solution: Correct Option B
Which of the following is a polar molecule?
Option A XeF4 Option B BF3 Option C SF4 Option D SiF4 Solution: Correct Option C Unsymmetrical distribution of the electron cloud leads to the formation of a polar molecule.
A hydrogen gas electrode is made by dipping platinum wire in a solution of HCI of pH = 10 and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of electrode would be?
Option A 1018 V Option B 0.059 V Option C 0.59 V Option D 0.118 V Solution: Correct Option C
Nitrobenzene on reaction with conc. 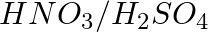 at 80-100°C forms which one of the following products?
at 80-100°C forms which one of the following products?
Option A 1, 2, 4-Trinitrobenzene Option B 1, 2-Dinitrobenzene Option C 1, 3- Dinitrobenzene Option D 1, 4- Dinitrobenzene Solution: Correct Option C Nitrobenzene on reaction with conc....
Some meta-directing substitutents in aromatic substitution are given. Which one is most deactivating?
Option A -NO2 Option B -C≡N Option C -SO3H Option D -COOH Solution: Correct Option A Deactivating power: -NO2 > -C ≡ N > -SO3H> COOH Thus, -NO2 is the most deactivating group because of the...
Roasting of sulphides gives the gas X as a byproduct. This is colourless gas with choking smell of brunt Sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as reducing agent and its acid has never been isolated. The gas X is:-
Option A SO3 Option B H2S Option C SO2 Option D CO2 Solution: Correct Option C We know that during the roasting of sulphide ore SO2 gas is obtained, reaction occurs as follows: 2M2S + 3O2 → 2M2O +...
Dipole induced dipole interactions are present in which of the following pairs:-
Option A Tetrafluro Silicon and He atoms Option B Water and alcohol Option C Chlorine and Carbon tetrachloride Option D HCI and He atoms Solution: Correct Option D Dipole-induced dipole...
A metal has a fcc lattice. The edge length of the unit cell is 404 pm. The density of the metal is 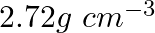 . The molar mass of the metal is:-
. The molar mass of the metal is:-
Option A 20g/mol Option B 40g/mol Option C 30g/mol Option D 27 g/mol Solution: Correct Option D The cell is fcc, so Z = 4 Edge length, a = 404 pm = 4.04×10-8 cm Density of metal, d = 2.72 g cm-3 NA...
Which of the following does not give oxygen on heating?
Option A (NH4)2Cr2O7 Option B KCIO3 Option C Zn(CIO3)2 Option D K2Cr2O7 Solution: Correct Option A All of the compounds are high in oxygen. With the exception of...
Reaction by which Benzaldehyde cannot be prepared:-
Solution: Correct Option A Option A is the Etard reaction, which produces benzaldehyde. The Rosenmund reduction procedure, which produces benzaldehyde, is option...
 as per the reaction. The reaction can go to completion by removing OH- ions by adding:
as per the reaction. The reaction can go to completion by removing OH- ions by adding:
Option A SO2 Option B HCI Option C KOH Option D CO2 Solution: Correct Option D A) SO2 = will react with water to form H2SO4 B) HCl = being acid it will reverse the reaction C) KOH = Being base it...
A magnetic moment of 1.73 BM will be shown by one among the following:-
Option A [CoCl6]4- Option B [Cu(NH3)4]2+ Option C [Ni(CN)4]2- Option D TiCI4 Solution: Correct Option B We are given that the magnetic moment 1.73 BM. $ \mu =\sqrt{n(n+2)} $ $...
If the equilibrium constant for N2 (g) + O2(g) ⇌ 2NO(g) is K, the equilibrium constant for 1 2 N2 (g) + 1 2 O2(g) ⇌ NO(g) will be:
(1) K (2) K2 (3) K1/2 (4) 1/2K Correct Answer: (3) K1/2 Explanation: Calculation - N2(g) + O2(g) 2NO(g); K ½ N2(g) + ½ O2(g) NO(g); K’ $\begin{array}{l} K =...
Antiseptics and disinfectants either kill or prevent growth of microganisms. Identify which of the following statements is not true:-
Option A Disinfectants harm the living tissues Option B A 0.2 % solution of phenol is an antiseptic while 1% solution acts as a disinfectant Option C Chlorine and Iodine are used as strong...
Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated Hl?
Solution: Correct Option D A carbocation is a reaction intermediate in this reaction, The order of stability of a carbocation is 3°>2°>1°.
Structure of the compound whose IUPAC name is 3-Ethyl-2-hydroxy-4-methylhex- 3- en-5-ynoic acid is:-
Solution: Correct Option C The structure of the compound which has the IUPAC designation 3-Ethyl-2-hydroxy-4-methyl...
The number of structural isomers possible from the molecular formula C3H9N is:
(1) 2 (2) 3 (3) 4 (4) 5 Correct Answer: (3) 4 Explanation:
Gadolinium belongs of 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
(1) [Xe]4f7 5d1 6s2 (2) [Xe]4f6 5d2 6s2 (3) [Xe]4f8 6d2 (4) [Xe]4f9 5s1 Correct Answer:(1) [Xe]4f7 5d1 6s2 Explanation: 64Gd = 54[Xe]6s2 4f7 5d1
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 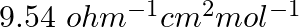 and at infinite dilution its molar conductance is
and at infinite dilution its molar conductance is 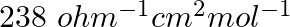 . The degree of ionization of ammonium hydroxide at the same concentration and temperature is:-
. The degree of ionization of ammonium hydroxide at the same concentration and temperature is:-
Option A 40.800% Option B 2.080% Option C 20.800% Option D 4.008% Solution: Correct Option D According to the question, Molar conductance at 0.1 M concentration,...
The formation of oxide ion, O2-(g), from oxygen atom requires first an exothermic and then an endothermic step as shown below:
O(g) + e- → O-(g) ; ΔfHo = −141 kJ mol-1 O-(g) + e- → O2-(g) ; ΔfHo = +780 kJ mol-1 Thus process of formation of O2- in gas phase is unfavorable though O2- is isoelectronic with neon. It is due to...
2,3-Dimethyl-2-butene can be prepared by heating which of the following compounds with a strong acid?
(1) (CH3)2C = CH – CH2 – CH3 (2) (CH3)2CH – CH2 − CH = CH2 (3) (4) (CH3)3CH − CH = CH2 Correct Answer: (4) (CH3)3CH − CH = CH2 Explanation:
A reaction having equal energies of activation for forward and reverse reactions has:-
Option A ΔH = ΔG = ΔS = 0 Option B ΔS = a Option C ΔG = 0 Option D ΔH = 0 Solution: Correct Option D $ We\,\,have:\,\,{{\left( Ea \right)}_{f}}={{\left( Ea \right)}_{b}} $ $ \Delta H={{\left( Ea...
Which of these is not a monomer for a high molecular mass silicone polymer?
Option A PhSiCl3 Option B Me2 SiCl2 Option C MeSiCl3 Option D Me3SiCl Solution: Correct Option D Because Me3SiCI only has one CI, it cannot form a silicon polymer with a high molecular mass. Because...
What is the maximum numbers of electrons that can be associated with the following set of quantum numbers? n = 3, l = 1 and m = −1
Option A 2 Option B 10 Option C 6 Option D 4 Solution: Correct Option A According to Pauli's exclusion principle, no two electrons can have all four quantum numbers the same. Thus one electron has...
An excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetraaqua chromium (II) chloride. The number of moles of AgCl precipitatede would be:-
Option A 0.01 Option B 0.001 Option C 0.002 Option D 0.003 Solution: Correct Option B The number of moles = Molarity × Volume = 0.01 × 0.1 Number of moles = 0.001 mol Hence, we can say that...
The number of carbon atoms per unit cell of the diamond is:
Option A 1 Option B 4 Option C 8 Option D 6 Solution: Correct Option C There are eight comer atoms, six face-centered atoms, and four additional atoms inside the diamond cubic unit cell. Therefore,...
Which of the following structure is similar to graphite?
Option A B2H6 Option B BN Option C B Option D B4C Solution: Correct Option B Boron nitride or (BN)x is known as the inorganic graphite and it has a structure similar to that of...
The basic structural unit of silicates is:
Option A SiO42- Option B SiO- Option C SiO44- Option D SiO32- Solution: Correct Option C We know that the silicates are the salts of silicic acid H4SiO4. The basic structural unit of...
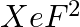 is isostructural with:
is isostructural with:
Option A BaCl2 Option B TeF2 Option ICl2- Option D SbCl3 Solution: Correct Option C Isostructural species are the species having the same number of bond pairs and lone pairs. There are 3 lone pairs...
Nylon is an example of:-
Option A Polythene Option B Polyester Option C Polysaccharide Option D Polyamide Solution: Correct Option D Nylon is formed by the condensation reaction of amines and carboxylic acid groups with...
The order of stability of the following tautomeric compounds is:
Option A II > III > I Option B I > II > III Option C III > II > I Option D II > I > III Solution: Correct Option C The order of tautomeric compound stability is...
In the reaction:
Solution: Correct Option D
Based on equation 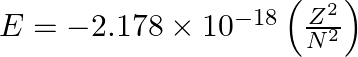 certain conclusions are written. Which of them is not correct?
certain conclusions are written. Which of them is not correct?
Option A For n = 1, the electron has more negative energy than it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit. Option B The negative sign in the...
The number of Faradays (F) required to produce 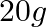 of calcium from molten
of calcium from molten 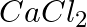 (Atomic mass of
(Atomic mass of 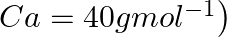 is
is
A 2
B 3
C 4
D 1
Correct Option D) As we know $$ \begin{aligned} & w = ZQ \\ & Q =\frac{ w }{ Z }=\frac{20}{40 / 2} F =1 F \end{aligned} $$ where $Z =\frac{ E }{ F }$
Which of the following is not correct about carbon monoxide?
A. The carboxyhemoglobin (haemoglobin bound to CO) is less stable than oxyhaemoglobin.
B. It is produced due to incomplete combustion.
C. It forms carboxyhaemoglobin.
D. It reduces oxygen carrying ability of blood
Correct Option A) Because CO is a stronger ligand than oxygen, carboxyhemoglobin is a more stable complex.
Which one of the following molecules contains no π bond?
Option A NO2 Option B CO2 Option C H2O Option D SO2 Solution: Correct Option C The central atom of oxygen has no d-orbitals for ????-bonding in the H2O molecule.
On electrolysis of dil. Sulphuric acid using platinum (Pt) electrode, the product obtained at anode will be: A. 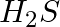 gas B.
gas B. 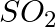 gas C. Hydrogen gas D. Oxygen gas
gas C. Hydrogen gas D. Oxygen gas
Correct Option: D) Solution: The reactions are as follows: Dissociation of sulfuric acid, $H _{2} SO _{4} \rightarrow 2 H ^{+}( aq )+ SO _{4}^{-2}( aq )$ Disssociation of water, $H _{2} O...
Which of the following lanthanoid ions is diamagnetic? (At. No., Ce = 58, Sm = 62, Vb = 70)
Option A Yb 2+ Option B Ce 2+ Option C Sm2+ Option D Eu2+ Solution: Correct Option A Diamagnetic means no unpaired electrons Ce (58) = Ce2+ = [Xe] 4f2 5d0 6s2, unpaired e⎺ = 2 Sm (62)= Sm2+ =...
Find out the solubility of 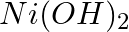 in
in 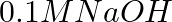 . Given that the ionic product of
. Given that the ionic product of 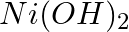 is
is 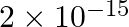
A 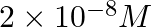
B 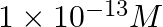
C 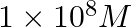
D 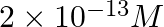
Correct Option D) Solution: $Ni ( OH )_{2}( s ) \leftrightharpoons Ni ^{+2}( aq )+2 OH ^{-}( aq )$ Thus we write the ionic product as, Ionic product $=\left[ Ni ^{+2}\right]\left[ OH...
For the reaction, 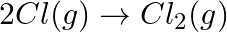 , the correct option is: A.
, the correct option is: A. 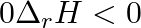 and
and 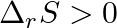 B.
B. 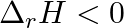 and
and 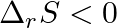 C.
C. 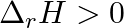 and
and 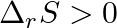 D.
D. 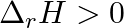 and
and 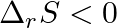
Correct Option: B) $2 Cl ( g ) \rightarrow Cl _{2}( g )$ As bond formation is accompanied by the release of energy $\therefore \Delta H =$-ve. Also, the number of particles decreases so $\Delta S =-...
How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3?
Option A 54. 0 conc. HNO3 Option B 45.0 conc. HNO3 Option C 90.0 conc. HNO3 Option D 70.0 conc. HNO3 Solution: Correct Option B
Hydrolysis of sucrose is given by the following reaction. Sucrose 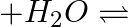 Glucose
Glucose 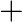 Fructose If the equilibrium constant
Fructose If the equilibrium constant 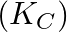 is
is 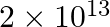 at
at 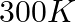 , the value of
, the value of 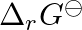 at the same temperature will be :
at the same temperature will be :
A 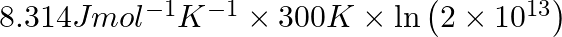
B 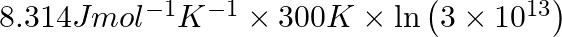
C 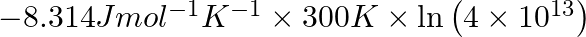
D 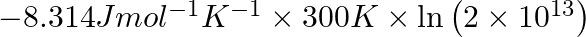
Correct Option D) The equation for the relationship between Gibbs energy and equilibrium constant is: $\Delta G ^{0}=- RT \ln K _{ c }$ $=-8.314 \times 300 \times \ln \left(2 \times...
Identify a molecule which does not exist. A. 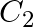 B.
B. 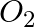 C.
C. 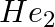 D.
D. 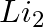
Correct Option: C) He atom electronic configuration – $1s_2$ The electronic configuration of the He 2 molecule is $\sigma 1 s ^{2} \sigma * 1 s ^{2}$ according to the linear combination of atomic...
Which of the following statements about the interstitial compounds is incorrect?
Option A They have higher melting points than the pure metal Option B They retain metallic conductive Option C They are chemically reactive Option D They are much harder than the pure metal...
The rate constant for a first order reaction 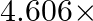
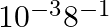 . The time required to reduce
. The time required to reduce 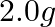 of the reactant to
of the reactant to 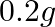 is: A.
is: A. 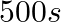 B.
B. 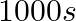 C.
C. 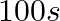 D.
D. 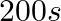
Correct Option: A) We calculate the time by $t =\frac{2.303}{ k } \log \frac{ A }{ A _{0}}=\frac{2.303}{4.606 \times 10^{-3}} \log \frac{2}{0.2}=500 sec$
The number of protons. Neutrons and electrons Lu. respectively, are:
A. 71, 71 and 104
B. 175, 104 and 71
C. 71, 104 and 71
D. 104, 71 and 71
Correct Option: D) Solution: ${ }_{71}^{175} Lu$ $n_{ p }= n _{e}=71$ $n _{ p }+ n _{ n }=175$ $n _{ n }=175-71=104$
