Solution: The difference in electronegativity between constituent atoms determines the ionic character of a molecule. As a result, the greater the difference, the greater the ionic character of a...
Arrange the bonds in order of increasing ionic character in the molecules: LiF,
Explain with the help of suitable example polar covalent bond.
Solution: The bond pair of electrons are not shared equally when two unique atoms with different electronegativities join to form a covalent bond. The bond pair is attracted to the nucleus of an...
Define electronegativity. How does it differ from electron gain enthalpy?
Solution: "Electronegativity refers to an atom's ability to attract a bond pair of electrons towards itself in a chemical compound." Sr. No Electronegativity Electron affinity 1 A tendency to...
Write the significance/applications of dipole moment.
Solution: There is a difference in electro-negativities of constituents of the atom in a heteronuclear molecule, which causes polarisation. As a result, one end gains a positive charge, while the...
Although both 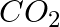 and
and 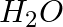 are triatomic molecules, the shape of the
are triatomic molecules, the shape of the 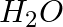 molecule is bent while that of
molecule is bent while that of 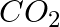 is linear. Explain this on the basis of dipole moment.
is linear. Explain this on the basis of dipole moment.
Solution: $CO_2$ has a dipole moment of 0 according to experimental results. And it's only possible if the molecule's shape is linear, because the dipole moments of the C-O bond are equal and...
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :(iii) Al and N.
Solution: Below is a list of Lewis symbols. To form a cation, a metal atom loses one or more electrons, while a nonmetal atom gains one or more electrons. Ionic bonds are formed between cations and...
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions : (i) K and S (ii) Ca and O
Solution: Below is a list of Lewis symbols. To form a cation, a metal atom loses one or more electrons, while a nonmetal atom gains one or more electrons. Ionic bonds are formed between cations and...
Write the resonance structures for 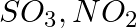 , and
, and 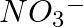
Solution: Resonance is the phenomenon that allows a molecule to be expressed in multiple ways, none of which fully explain the molecule's properties. The molecule's structure is called a resonance...
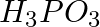 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing
can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing 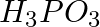 ? If not, give reasons for the same.
? If not, give reasons for the same.
Solution: The positions of the atoms remain constant in canonical forms, but the positions of the electrons change. The positions of atoms change in the given canonical forms. As a result, they...
Explain the important aspects of resonance with reference to the 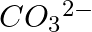 ion.
ion.
Solution: However, while the carbonate ion cannot be represented by a single structure, the properties of the ion can be described by two or more different resonance structures. The actual structure...
Define Bond length.
Solution: Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
How do you express the bond strength in terms of bond order?
Solution: During the formation of a molecule, the extent of bonding that occurs between two atoms is represented by the bond strength of the molecule. As the bond strength increases, the bond...
Although geometries of 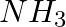 and
and 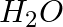 molecules are distorted tetrahedral, bond angle in water is less than that of Ammonia. Discuss.
molecules are distorted tetrahedral, bond angle in water is less than that of Ammonia. Discuss.
Solution: Ammonia's central atom (N) has one lone pair and three bond pairs. In water, the central atom (O) has two lone pairs and two bond pairs. As a result, the two bond pairs repel the two lone...
Discuss the shape of the following molecules using the VSEPR model: 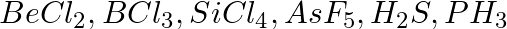
Solution: $BeCl_2$ The central atom does not have a lone pair, but it does have two bond pairs. As a result, its shape is AB2, or linear. $BCl_3$ The central atom has three bond pairs but no lone...
Write the favourable factors for the formation of an ionic bond.
Solution: Ionic bonds are formed when one or more electrons are transferred from one atom to another. As a result, the ability of neutral atoms to lose or gain electrons is required for the...
Define the octet rule. Write its significance and limitations
Solution: “Atoms can combine either by transferring valence electrons from one atom to another or by sharing their valence electrons in order to achieve the closest inert gas configuration by having...
Draw the Lewis structures for the following molecules and ions : 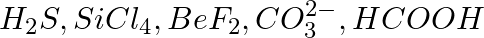
Solution: The lewis dot structures are:
Write Lewis symbols for the following atoms and ions: Sand 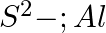 and
and 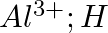 and
and 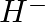
Solution: For S and S2- A sulphur atom has only 6 valence electrons, which is a very small number. As a result, the Lewis dot symbol for the letter S is The presence of a...
Write Lewis dot symbols for atoms of the following elements :e) N f) Br
Solution: Nitrogen atoms have only five valence electrons in total. As a result, the Lewis dot symbol for N is Bromine, because the atom has only seven valence electrons. As a result,...
Write Lewis dot symbols for atoms of the following elements :c) B d) O
Solution: Boron atoms have only three valence electrons, which is a very small number. As a result, the Lewis dot symbols for B are as follows: The oxygen atom has only six valence...
Write Lewis dot symbols for atoms of the following elements :
a) Mg
b) Na
Solution: Only two valence electrons exist in the magnesium atom. As a result, the Lewis dot symbols for Mg are as follows: Only one valence electron exists in the sodium atom. As a...
Explain the formation of a chemical bond.
Answer: "A chemical bond is an attractive force that holds a chemical species' constituents together." For chemical bond formation, many theories have been proposed, including valence shell electron...
Is there any change in the hybridisation of B and N atoms as a result of the following reaction? 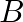 and
and 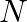
![Rendered by QuickLaTeX.com \[BF _{3}+ NH _{3} \rightarrow F _{3} B \cdot NH _{3}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f87a2a8a2c94b67d7949b58594c840a9_l3.png)
Ans.)
Answer: F3B.NH3 is a reaction product formed by the reaction of BF3 + NH3. As a result of changing the hybridization of the B-atom to sp3, the product NH3 is produced. The hybridization of N-atoms,...
Describe the change in hybridisation (if any) of the Al atom in the following reaction. ![Rendered by QuickLaTeX.com \[AlCl _{3}+C l^{-} \rightarrow A l C l_{4}^{-}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-6a3539afdf8fa241be293a500542ae7d_l3.png)
Answer: The hybridization of Al atom alters from $sp ^{2}$ in $AlCl _{3}$ to $sp ^{3}$ in $AlCl _{4}^{-}$. In $AlCl _{3}$, Al contains 3 bond pairs of electrons and zero lone pair of electrons....
What is meant by hybridisation of atomic orbitals? Describe the shapes of 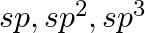 hybrid orbitals.
hybrid orbitals.
Answer: Intermixing orbitals of various energies to redistribute their energy and create new orbitals with equal energies and forms. The new orbitals are hybridized. In this way, two s orbitals...
Which out of 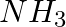 and
and 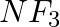 has higher dipole moment and why?
has higher dipole moment and why?
Answer: When comparing dipole moments, ammonia (1.47D) has a higher dipole moment than $NF_3$. (0.24D). Both molecules have a pyramidal molecular geometry, which is characteristic of this type of...
Displacement versus time curve for a particle executing SHM is shown. Identify the points marked at which
i) velocity of the oscillator is zero ii) speed of the oscillator is maximum Answer: (i) The oscillator's velocity is zero when the points A, C, E, and G are at their extreme positions. (ii) The...
Explain why BeH2 molecule has a zero dipole moment although the Be–H bonds are polar.
Answer: The dipole moment of a molecule with symmetrical and linear geometries is zero because they are vector in nature and the dipole of different bonds cancel with one another, whereas the dipole...
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar.
Answer: The square planar geometry has a bond angle of 90 degrees with the equilateral triangle geometry. In comparison to the tetrahedral bond angle, which is 109.5 0, this is less favourable. This...
Arrange the bonds in order of increasing ionic character in the molecules: LiF, 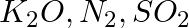 , and
, and 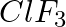 .
.
Answer: The differential in electronegativity between the atoms that make up a molecule determines the ionic character of that molecule. As a result, the greater the difference between two...
Explain with the help of suitable example polar covalent bond.
Answer: When two separate atoms join to form a covalent connection, their electrons are not shared equally. A more electronegative atom's nucleus attracts the bond pair. In this case, an...
Define electronegativity. How does it differ from electron gain enthalpy?
Answer: Sr. No Electronegativity Electron affinity 1 The electronegativity of an atom in a chemical compound refers to its proclivity to attract the shared pairs of electrons that are present in the...
Write the significance/applications of dipole moment.
Answer: The difference in electro-negativities of atom components causes polarisation in heteronuclear molecules. So one end gets a positive charge and the other gets a negative charge. Molecules...
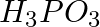 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing
can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing 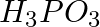 ? If not, give reasons for the same.
? If not, give reasons for the same.
Answer: The positions of the atoms remain constant in the canonical forms, while the positions of the electrons change. The positions of atoms shift in the specified canonical forms. As a result,...
Explain the important aspects of resonance with reference to the 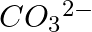 ion.
ion.
Answer: Although the properties of the carbonate ion cannot be described by a single structure, they can be described by two or more resonance structures. The carbonate ion's true structure is a...
Define Bond length.
Answer: “The equilibrium distance between the nuclei of two bound atoms in a molecule is defined as bond length.”
How do you express the bond strength in terms of bond order?
Answer: During the formation of a molecule, the extent of bonding that happens between two atoms is represented by the bond strength of the molecule. As the bond strength develops, the bond becomes...
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of Ammonia. Discuss.
Answer: Ammonia's central atom (N) has 1 lone pair and 3 bond pairs. Water's central atom (O) has two lone pairs and two bond pairs. The two lone pairs on the O- atom of water reject the two bond...
Write the favourable factors for the formation of an ionic bond.
Answer: An ionic link is formed by moving electrons from one atom to another. This means that ionic bond formation is dependent on neutral atom flexibility. The lattice energy of the molecule...
Define the octet rule. Write its significance and limitations
Answer: "Atoms can combine either by transferring or sharing valence electrons to achieve the closest inert gas configuration," says the Octet Rule. Octet rule describes how chemical bonds occur...
The types of hybrid orbitals of nitrogen in NO2+, NO3- and NH4+respectively are expected to be
(i) sp, sp3 and sp2
(ii) sp, sp2 and sp3
(iii) sp2, sp and sp3
(iv) sp2, sp3 and sp
Solution: Option (ii) is the answer. The hybridisation of each molecule gives us an idea about the hybrid orbitals.
Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs. (i) [NF3 and BF3] (ii) [BF4- and NH4+] (iii) [BCl3 and BrCl3] (iv) [NH3 and NO3-]
From a structural standpoint, we can see that, NF3 is pyramidal whereas BF3 is planar triangular. BF4- and NH4+ ions are tetrahedral in structure. BCl3 is triangular planar and BrCl3 is...
