Frequency of radiation $(\nu)$, $\nu=\frac{1}{2.0 \times 10^{-9} s}$ $\nu=5.0 \times 10^{8} s^{-1}$ Energy $(E)$ of source $=$ Nhv Where, $N$ is the no. photons emitted $\mathrm{h}$ is Planck's...
Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nanosecond range. If the radiation source has the duration of
Arrange the following type of radiations in increasing order of frequency: (a) radiation from microwave oven (b) amber light from traffic signal (c) radiation from FM radio (d) cosmic rays from outer space and (e) X-rays.
The following is the frequency order in ascending order: Radiation from FM radio < amber light < radiation from microwave oven < X- rays < cosmic rays The following is the increasing...
Explain, giving reasons, which of the following sets of quantum numbers are not possible.
a) 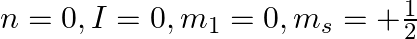
b) 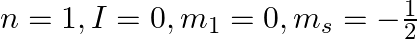
c) 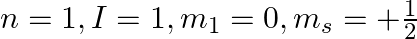
d) 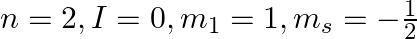
e) 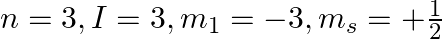
f) 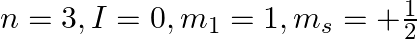
a) This is not possible. The number n cannot be zero. (b) Possible. (c) This is not possible. The value of l can't be the same as the value of n. (d) This is not possible. Because mt can't be 1 when...
What is the lowest value of n that allows g orbitals to exist?
For g-orbitals, l = 4. The possible values of ‘l’ range from 0 to (n-1),. For any given value of ‘n’, Hence, least value of n = 5, l = 4 (g orbital),
(II) What are the atomic numbers of elements whose outermost electrons are represented by (a) 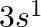 (b)
(b) 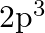 and (c)
and (c) 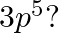
(II) (a) $3 \mathrm{~s}^{1}$ Complete electronic configuration: $1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}^{6} 3 \mathrm{~s}^{1}$ Total no. electrons in the atom $=2+2+6+1=11 \quad...
The electron energy in hydrogen atom is given by 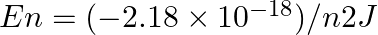 . Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength of light in cm that can be used to cause this transition?
. Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength of light in cm that can be used to cause this transition?
Required energy for the ionization from $\mathrm{n}=2$ is: $ \begin{array}{l} \Delta E=E_{\infty}-E_{2} \\ =\left[\left(\frac{-\left(2.18 \times...
What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the light emitted when the electron returns to the ground state? The ground state electron energy is 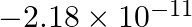 ergs. The ground-state electron energy is
ergs. The ground-state electron energy is 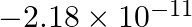 ergs.
ergs.
$ E_{5}=\frac{-\left(2.18 \times 10^{-18}\right) Z^{2}}{(n)^{2}} $ Where, $Z$ denotes the atom's atomic number Ground state energy $=-2.18 \times 10^{-11}$ ergs $=-2.18 \times 10^{-11} \times...
Calculate the wavenumber for the longest wavelength transition in the Balmer series of atomic hydrogen.
The Balmer series of the hydrogen emission spectrum, ni = 2. Hence, wavenumber expression ν is: $ \bar{\nu}=\left[\frac{1}{(2)^{2}}-\frac{1}{n_{f}^{2}}\right]\left(1.097 \times 10^{7}...
How can the production of dihydrogen, obtained from ‘coal gasification’, be increased?
Solution: By the course of coal gasification, dihydrogen is created as $C_{(g)}+H_{2} O_{(g)} \rightarrow C O_{(g)}+H_{2(g)}$ [C-Coal] Response with carbon monoxide with steam within the sight of an...
The equilibrium constant expression for a gas reaction is, ![Rendered by QuickLaTeX.com K_{c}=\frac{\left[N H_{3}{ }^{4}\left[\mathrm{O}_{2}\right]^{5}\right.}{[\mathrm{NO}]^{4}\left[\mathrm{H}_{2} \mathrm{O}\right]^{6}}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-951fdaf6f5740b9392c024eddb4c57da_l3.png) Write the balanced chemical equation corresponding to this expression.
Write the balanced chemical equation corresponding to this expression.
Answer: The equilibrium constant, Kc, is defined as the product of the equilibrium concentrations of products over the equilibrium concentrations of reactants, each raised to the power of the...
A mixture of 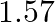 mol of
mol of 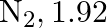 mol of
mol of 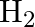 and
and 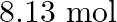 of
of 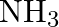 is introduced into a
is introduced into a 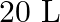 reaction vessel at
reaction vessel at 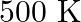 . At this temperature, the equilibrium constant,
. At this temperature, the equilibrium constant, 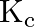 for the reaction
for the reaction 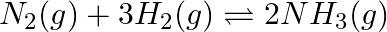 is
is  Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
Answer: The given reaction is: $\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g})$ The given concentration of various species is...
A sample of 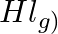 is placed in a flask at a pressure of
is placed in a flask at a pressure of 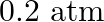 . At equilibrium, the partial pressure of
. At equilibrium, the partial pressure of 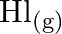 is
is 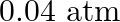 .What is
.What is 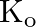 for the given equilibrium?
for the given equilibrium? 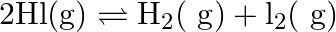
Answer: The initial concentration of $HI$ is $0.2 atm$. It has a partial pressure of $0.04 atm$ when it is in equilibrium with the surrounding environment. The pressure of $HI$ drops by...
For the following equilibrium, 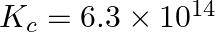 at
at 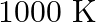
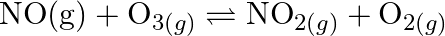 Both the forward and reverse reactions in the equilibrium are elementary bimolecular reactions. What is
Both the forward and reverse reactions in the equilibrium are elementary bimolecular reactions. What is 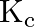 , for the reverse reaction?
, for the reverse reaction?
Answer: Kp and Kc are equilibrium constants for reversible reactions. The equilibrium constant Kp is stated in terms of atmospheric pressure, whereas Kc is expressed in terms of concentrations...
Write the expression for the equilibrium constant, 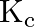 for each of the following reactions: (i)
for each of the following reactions: (i) 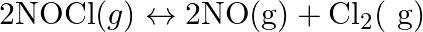 (ii)
(ii) 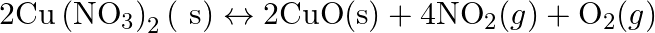
Answer: The equilibrium constant, Kc, is defined as the product of the equilibrium concentrations of products over the equilibrium concentrations of reactants, each raised to the power of the...
Explain the formation of a chemical bond.
Answer: “A chemical bond is an attractive force that binds chemical elements together.” Many theories exist for forming chemical bonds, including valence shell electron pair repulsion, electronic,...
The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. If any of these combination(s) has/have the same energy lists: n = 4, l = 2, ml = –2 , ms = –1/2 n = 3, l = 2, ml= 1 , ms = +1/2 n = 4, l = 1, ml = 0 , ms = +1/2 n = 3, l = 2, ml = –2 , ms = –1/2 n = 3, l = 1, ml = –1 , ms= +1/2 n = 4, l = 1, ml = 0 , ms = +1/2
The 4d, 3d, 4p, 3d, 3p, and 4p orbitals are home to electrons 1, 2, 3, 4, 5, and 6. (respectively). Ranking these orbitals in the increasing order of energies: (3p) < (3d) < (4p) < (4d).
The unpaired electrons in Al and Si are present in 3p orbital. Which electrons will experience more effective nuclear charge from the nucleus?
The net positive charge acting on an electron in an atom's orbital with more than one electron is known as the nuclear charge. The nuclear charge increases as the atomic number increases. Silicon...
List gases which are responsible for greenhouse effect.
The major gases that cause greenhouse effect are: 1) Chlorofluorocarbons (CFCs) 2) Methane (CH4) 3) Carbon dioxide (CO2) 4) Nitrous oxide (NO) 5) Water(H2O) 6) Ozone (O3)
Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Carbon dioxide (CO2) and carbon monoxide (CO) are both produced when various fuels are burned. In nature, carbon monoxide is harmful, but carbon dioxide is non-toxic. Because carbon monoxide forms a...
Calculate the wavelength, frequency and wavenumber of a light wave whose period is 2.0 × 10–10 s.
Frequency of the light wave $\nu$ = $\frac{1}{Period} \frac{1}{Period}$ $=\frac{1}{2.0\times 10^{-10}\, s} =5.0\times 10^{9}\, s^{-1 }$ Wavelength of the light wave$\lambda=c\nu$ Where, c denotes...
How many neutrons and protons are there in the following nuclei? 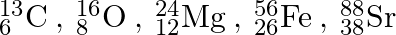
\({}_{6}^{13}C\): Mass number of carbon-13 = 13 Atomic number of carbon = Number of protons in one carbon atom = 6 Therfore, total number of neutrons in 1 carbon atom = Mass number – Atomic number =...
(i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons.
1 electron weighs 9.109*10-31 kg. Therefore, number of electrons that weigh 1 g (10-3 kg) = 1.098*1027 electrons (ii) Mass of one mole of electrons = NA* mass of one electron =...
Convert the following into basic units: (i) 28.7 pm (ii) 15.15 pm (iii) 25365 mg
(i) 28.7 pm $1 pm = 10^{ -12 } \; m$ $28.7 pm = 28.7 \times 10^{ -12 } \; m$ $= 2.87 \times 10^{ -11 } \; m$ (ii) 15.15 pm $1 pm = 10^{ -12 } \; m$ $15.15 pm = 15.15 \times 10^{ -12 } \; m$...
A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. A volume of 10.0 L (measured at STP) of this welding gas is found to weigh 11.6 g. Find: (i) Empirical formula (ii) Molar mass of the gas, and (iii) Molecular formula
(i) Empirical formula 1 mole of $CO_{ 2 }$ contains 12 g of carbon Therefore, 3.38 g of $CO_{ 2 }$ will contain carbon = $\frac{ 12 \; g }{ 44 \; g } \; \times 3.38 \; g$ = 0.9217 g 18 g of...
Calculate the concentration of nitric acid in moles per litre in a sample which has a density, 1.41 g mL–1 and the mass per cent of nitric acid in it being 69%
Mass percent of HNO3 in sample is 69 % Thus, 100 g of HNO3 contains 69 g of HNO3 by mass. Molar mass of HNO3 = { 1 + 14 + 3(16)} g.mol^{-1}g.mol−1 = 1 + 14 + 48 = 63g mol^{-1}=63gmol−1 Now,...
Assertion (A): Toluene on Friedel Crafts methylation gives o– and p–xylene. Reason (R): CH3-group bonded to benzene ring increases electron density at o– and p– position. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct. (iv) A is not correct but R is correct.
Option (i) is correct
Match the reactions given in Column I with the reaction types in Column II.
(i) is d (ii) is a (iii) is b (iv) is c
Pressure versus volume graph for a real gas and an ideal gas is shown in Fig. 5.4. Answer the following questions based on this graph. (i) Interpret the behaviour of real gas with respect to an ideal gas at low pressure. (ii) Interpret the behaviour of real gas with respect to an ideal gas at high pressure. (iii)Mark the pressure and volume by drawing a line at the point where real gas behaves as an ideal gas.
(i) At low pressure as the dark blue curve and the sky blue curve are approaching each other, it shows that the real gas is behaving as an ideal gas at a low pressure. (ii) At high pressure as the...
The variation of pressure with the volume of the gas at different temperatures can be graphically represented as shown in Fig. 5.3. Based on this graph answer the following questions. (i) How will the volume of a gas change if its pressure is increased at constant temperature? (ii) At constant pressure, how will the volume of a gas change if the temperature is increased from 200K to 400K?
(i) As the temperature is constant, and the pressure is increasing and the change in the volume is seen as exponentially decreasing. (ii) At constant pressure, by increasing the temperature there is...
Explain the effect of increasing the temperature of a liquid, on intermolecular forces operating between its particles, what will happen to the viscosity of a liquid if its temperature is increased?
As the temperature increases, the intermolecular force operating between the particles decreases, the bond strength increases and also the kinetic energy increases. Hence, as the temperature...
The relation between the pressure exerted by an ideal gas (Pideal) and observed pressure (Pearl) is given by the equation: Pideal = Preal+ an2/V2 If the pressure is taken in Nm-2, the number of moles in mol and volume in m3, Calculate the unit of ‘a’. What will be the unit of ‘a’ when pressure is in atmosphere and volume in dm3?
We know that: Pideal = Preal + an2/V2 Pideal – Preal= an2/V2 Nm-2 = a*mol2/m6 A = Nm4mol-2 The unit of ‘a’ when the pressure is taken in Nm-2, number of moles in “mol” and volume in m3 is Nm4mol-2...
For real gases the relation between p, V and T are given by van der Waals equation: [(P + an2) / V2](V – nb) = nRT Where‘a’ and ‘b’ are van der Waals constants, ‘nb’ is approximately equal to the total volume of the molecules of a gas. ‘a’ is the measure of the magnitude of intermolecular attraction. (i) Arrange the following gases in the increasing order of ‘b’. Give reason. O2, CO2, H2, He (ii) Arrange the following gases in the decreasing order of magnitude of ‘a’. Give reason. CH4, O2, H2
(i) The increasing order of ‘b’ is as follows: He < H2< O2< CO2. As the Vander Waals constant ‘b’ is approximately equal to the total volume of the molecules of a gas. (ii)The decreasing...
The addition of HBr to 1-butene gives a mixture of products A, B and C;The mixture consists of (i) A and B as major and C as minor products (ii) B as major, A and C as minor products (iii) B as minor, A and C as major products (iv) A and B as minor and C as major products
Option I is the correct response.
Assertion (A): Excessive use of chlorinated synthetic pesticides causes soil and water pollution. Reason (R): Such pesticides are non-biodegradable. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct. (iv) A is not correct but R is correct
Option (i) is the answer.
Based on chemical reactions involved, explain how chlorofluorocarbons cause thinning of the ozone layer in the stratosphere
The action of ultraviolet radiation causes chlorofluorocarbons to dissociate, releasing the free chlorine radicle. U V radiations + CF2Cl2 + Cl + CFC Now that this Chlorine radicle has formed, it is...
Biochemical Oxygen Demand, (BOD) is a measure of organic material present in water. BOD value less than 5 ppm indicates a water sample to be __________. (i) rich in dissolved oxygen. (ii) poor in dissolved oxygen. (iii) highly polluted. (iv) not suitable for aquatic life.
The solution is option (i).
Which of the following statements is not true about classical smog? (i) Its main components are produced by the action of sunlight on emissions of automobiles and factories. (ii) Produced in a cold and humid climate. (iii) It contains compounds of reducing nature. (iv) It contains smoke, fog and sulphur dioxide.
The solution is option (i).
Photochemical smog occurs in a warm, dry and sunny climate. One of the following is not amongst the components of photochemical smog, identify it. (i) NO2 (ii) O3 (iii) SO2 (iv) Unsaturated hydrocarbon
The solution is option (iii).
Which of the following gases is not a greenhouse gas? (i) CO (ii) O3 (iii) CH4 (iv) H2O vapour
Option (i) is the correct answer
The critical temperature (Tc) and critical pressure (Pc) of CO2 are 30.98°C and 73atm respectively. Can CO2(g) be liquefied at 32°C and 80atm pressure?
CO2 gas cannot be liquefied at a temperature which is greater than its critical temperature i.e 30.98°C even by applying any pressure. So as the given temperature is 32°C by applying a pressure of...
Compressibility factor, Z, of a gas is given as Z = PV/ nRT (i) What is the value of Z for an ideal gas? (ii) For real gas what will be the effect on the value of Z above Boyle’s temperature?
(i) Compressibility factor, Z is defined as the ratio of the product of pressure and volume to the product of the number of moles, gas constant and temperature. For an ideal gas, the value of Z is...
One of the assumptions of the kinetic theory of gases is that there is no force of attraction between the molecules of a gas. State and explain the evidence that shows that the assumption is not applicable for real gases.
Under a condition of low pressure and high temperature the assumption made by kinetic theory is true. At high temperature, the molecules will be very far from each other and at low pressure, the...
Name two intermolecular forces that exist between HF molecules in a liquid state.
Hydrogen bonding and dipole-dipole interaction (HF-HF interaction) exists between HF molecule in a liquid state.
Name the energy which arises due to the motion of atoms or molecules in a body. How is this energy affected when the temperature is increased?
Thermal energy arises due to the motion of particles (atoms or molecules) in the body. If we increase the temperature then the kinetic energy of atom and molecule increases significantly and they...
The pressure exerted by saturated water vapour is called aqueous tension. What correction term will you apply to the total pressure to obtain a pressure of dry gas?
The total pressure of the gas is Pmoist gas = Pdry gas By applying the correction term, we have: Pdry gas = Pmoist gas – Aqueous tension Therefore, the correction term applied to the total pressure...
Physical properties of ice, water and steam are very different. What is the chemical composition of water in all three states?
H2O exists in three different states of matter. It exists in the solid form as ice, in the liquid form as water and as steam in the gaseous state. All of these states consist of water, due to which...
Which of the following figures does not represent 1 mole of dioxygen gas at STP? (i) 16 grams of gas (ii) 22.7 litres of gas (iii) 6.022 × 1023 dioxygen molecules (iv) 11.2 litres of gas
The correct options are (i) and (iv).
With regard to the gaseous state of matter which of the following statements are correct? (i) Complete order of molecules (ii) Complete disorder of molecules (iii) Random motion of molecules (iv) Fixed position of molecules
Option (ii) and (iii) are the correct statements.
How does the surface tension of a liquid vary with an increase in temperature? (i) Remains the same (ii) Decreases (iii) Increases (iv) No regular pattern is followed
The correct option is (ii) Decreases.
Increase in kinetic energy can overcome intermolecular forces of attraction. How will the viscosity of liquid be affected by the increase in temperature? (i) Increase (ii) No effect (iii) Decrease (iv) No regular pattern will be followed
The correct option is (iii) Decrease.
Which curve in Fig. 5.2 represents the curve of an ideal gas? (i) B only (ii) C and D only (iii) E and F only (iv) A and B only
The correct option is (i) B only.
Atmospheric pressures recorded in different cities are as follows: Cities Shimla Bangalore Delhi Mumbai p in N/m2 1.01×105 1.2×105 1.02×105 1.21×105. Consider the above data and mark the place at which liquid will boil first. (i) Shimla (ii) Bangalore (iii) Delhi (iv) Mumbai
The correct option is (i) Shimla
What is the SI unit of viscosity coefficient (η)? (i) Pascal (ii) Nsm–2 (iii) km–2 s (iv) N m–2
The correct option is (ii) Nsm–2
Gases possess characteristic critical temperature which depends upon the magnitude of intermolecular forces between the particles. Following are the critical temperatures of some gases. Gases H2 He O2 N2 Critical temperature in Kelvin 33.2 5.3 154.3 126 From the above data what would be the order of liquefaction of these gases? Start writing the order from the gas liquefying first (i) H2, He, O2, N2 (ii) He, O2, H2, N2 (iii) N2, O2, He, H2 (iv) O2, N2, H2, He
The correct option is (iv) O2, N2, H2, He.
As the temperature increases, the average kinetic energy of molecules increases. What would be the effect of the increase of temperature on pressure provided the volume is constant? (i) increases (ii) decreases (iii) remains the same (iv) becomes half
The correct option is (i) increases.
The pressure of a 1:4 mixture of dihydrogen and dioxygen enclosed in a vessel is one atmosphere. What would be the partial pressure of dioxygen? (i) 0.8×105 atm (ii) 0.008 Nm–2 (iii) 8×104 Nm–2 (iv) 0.25 atm
The correct option is (iii) 8×104 Nm–2
Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of dipoles possess ‘partial charges’. The partial charge is (i) more than unit electronic charge (ii) equal to unit electronic charge (iii) less than unit electronic charge (iv) double the unit electronic charge
The correct option is (iii) less than unit electronic charge.
The interaction energy of the London force is inversely proportional to the sixth power of the distance between two interacting particles but their magnitude depends upon (i) charge of interacting particles (ii) mass of interacting particles (iii) polarisability of interacting particles (iv) strength of permanent dipoles in the particles.
The correct option is (iii) polarisability of interacting particles.
A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in Fig. 5.1. Which of the following order of pressure is correct for this gas? (i) p1 > p2 > p3 > p4 (ii) p1 = p2 = p3 = p4 (iii) p1 < p2 < p3 < p4 (iv) p1 < p2 = p3 < p4
The correct option is (iii) p1 < p2 < p3 < p4.
A person living in Shimla observed that cooking food without using pressure cooker takes more time. The reason for this observation is that at high altitude: (i) pressure increases (ii) temperature decreases (iii) pressure decreases (iv) temperature increases
The correct option is (iii) pressure decreases.
Match the intermediates given in Column I with their probable structure in
(i) is a (ii) is a (iii) is b
If a liquid compound decomposes at its boiling point, which method(s) can you choose for its purification. It is known that the compound is stable at low pressure, steam volatile and insoluble in water.
Because the liquid component decomposes near its boiling point, indicating that it is heat-sensitive, we purify it using "Steam distillation." For temperature-sensitive materials, this is done.
Which of the following compounds contain all the carbon atoms in the same hybridisation state? (i) H—C ≡ C—C ≡ C—H (ii) CH3—C ≡ C—CH3 (iii) CH2 = C = CH2 (iv) CH2 = CH—CH = CH2
The correct answers are I and (iv).
Ionic species are stabilised by the dispersal of charge. Which of the following carboxylate ion is the most stable?
Option IV is the correct answer.
During the hearing of a court case, the judge suspected that some changes in the documents had been carried out. He asked the forensic department to check the ink used at two different places. According to you which technique can give the best results? (i) Column chromatography (ii) Solvent extraction (iii) Distillation (iv) Thin-layer chromatography
Option IV is the correct answer.
Electronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, the carbon marked with an asterisk is most electronegative? (i) CH3 – CH2 – *CH2 –CH3 (ii) CH3 – *CH = CH – CH3 (iii) CH3 – CH2 – C ≡ *CH (iv) CH3 – CH2 – CH = *CH2
Option (iii) is the correct answer.
Which of the following is the correct IUPAC name? (i) 3-Ethyl-4, 4-dimethylheptane (ii) 4,4-Dimethyl-3-ethylheptane (iii) 5-Ethyl-4, 4-dimethylheptane (iv) 4,4-Bis(methyl)-3-ethylheptane
Option I is the correct answer.
Assertion (A): Silicons are water-repelling in nature. Reason (R): Silicons are organosilicon polymers, which have (–R2SiO–) as repeating unit. (i) A and R both are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) A and R both are not true. (iv) A is not true but R is true.
Correct Option is (ii)
Assertion (A): If aluminium atoms replace a few silicon atoms in three the dimensional network of silicon dioxide, the overall structure acquires a negative charge. Reason (R): Aluminium is trivalent while silicon is tetravalent. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct (iv) A is not correct but R is correct.
Correct Option is (i)
Match the species given in Column I with the properties mentioned in Column II.
(i) is e (ii) is c (iii) is d (iv) is a,b
Identify the compounds A, X and Z in the following reactions : (i) A + 2HCL + 5H2O → 2NaCI + X X → HBO2 → Z
A is Borax, which produces Orthoboric acid when it combines with HCl in the presence of water (X). When Orthoboric acid is heated, Metaboric is formed, and when heated further, the chemical Z, i.e....
Explain the following : (ix) BF3 does not hydrolyse. (x) Why does the element silicon, not form graphite-like structure whereas carbon does.
(ix) BF3 does not entirely hydrolyze. Instead, it forms boric acid and fluoroboric acid after partial hydrolysis. Because the HF is produced first, it interacts with H3BO3. As a result, BF3 does not...
Explain the following : (iii) Aluminium forms [AlF6]3- ion but boron does not form [BF6]3- ion. (iv) PbX2 is more stable than PbX4.
(iii) While aluminium has an empty d-orbital to accommodate the electrons from the fluorine atom, boron does not have an empty d-orbital. (iv); Pb is a part of the periodic table's group 14. (carbon...
When BCl3 is treated with water, it hydrolyses and forms [B[OH]4]- only whereas AlCl3 in acidified aqueous solution forms [Al (H2O)6]3+ ion. Explain what is the hybridisation of boron and aluminium in these species?
AlCl3 + 6H2O → [Al(H2O)6]3+ + 3Cl– The 6 H2O molecules bind to Al, donating 6 electron pairs to the Al3+ ion's 3s, 3p, and 3d orbitals. As a result, the Al atom hybridization in [Al(H2O)6]3+ species...
If a trivalent atom replaces a few silicon atoms in a three-dimensional network of silicon dioxide, what would be the type of charge on an overall structure?
One valence electron of each Si atom will become free if a few tetrahedral Si atoms in a three-dimensional network structure of SiO2 are replaced with an equal number of trivalent atoms. As a...
Explain the following : (i) CO2 is a gas whereas SiO2 is solid. (ii) Silicon forms SiF62- ion whereas the corresponding fluoro compound of carbon is not known.
I In comparison to carbon, silicon has a huge size. It does not provide a decent overlapping pattern. With oxygen atoms, it forms four single covalent bonds. Two Si atoms are connected to each...
Explain why the following compounds behave as Lewis acids? (ii) AlCl3
Because aluminium contains three electrons in its valence shell, AlCl3 forms a covalent connection with chlorine by creating three single chlorine bonds, making it an electron-deficient molecule and...
Identify the correct resonance structures of carbon dioxide from the ones given below : (i) O – C ≡ O (ii) O = C = O (iii) –O ≡ C – O+ (iv) –O – C ≡ O+
The solutions are options (ii) and (iv).
Ionisation enthalpy (∆i H1 kJ mol–1) for the elements of Group 13 follows the order. (i) B > Al > Ga > In > Tl (ii) B < Al < Ga < In < Tl (iii) B Ga Tl (iv) B > Al In < Tl
Option IV is the correct response
Catenation i.e., linking of similar atoms dependson size and electronic configuration of atoms. The tendency of catenation in Group 14 elements follows the order: (i) C > Si > Ge > Sn (ii) C >> Si > Ge ≈ Sn (iii) Si > C > Sn > Ge (iv) Ge > Sn > Si > C
Option II is the correct response.
Boric acid is an acid because its molecule (i) contains replaceable H+ ion (ii) gives up a proton (iii) accepts OH– from water releasing a proton (iv) combines with a proton from the water molecule
Option III is the correct response.
The exhibition of the highest co-ordination number depends on the availability of vacant orbitals in the central atom. Which of the following elements is not likely to act as a central atom in MF6 3–? (i) B (ii) Al (iii) Ga (iv) In
Option I is the correct response.
W hich of the following oxides is acidic? (i) B2O3 (ii) Al2O3 (iii) Ga2O3 (iv) In2O3
Option I is the correct response.
Which of the following is a Lewis acid? (i) AlCl3 (ii) MgCl2 (iii) CaCl2 (iv) BaCl2
Option I is the correct response.
How many neutrons and protons are there in the following nuclei?
Ans: 24 is the mass number. The number of protons in an atom equals the number of atoms in the atom. Mass number – Atomic number = 24 – 12 = 12 neutrons Numerical value of mass nubmer = 56 Number...
Assertion (A): The black body is an ideal body that emits and absorbs radiations of all frequencies. Reason (R): The frequency of radiation emitted by a body goes from a lower frequency to higher frequency with an increase in temperature.
(i) Both A and R are true and R is the correct explanation of A. (ii) Both A and R are true but R is not the explanation of A. (iii) A is true and R is false. (iv) Both A and R are false. ...
The effect of the uncertainty principle is significant only for the motion of microscopic particles and is negligible for the macroscopic particles. Justify the statement with the help of a suitable example.
The uncertainty principle is applicable only for microscopic particles and can be concluded from the uncertainty measurement. Example: Take a particle of mass = 1 milligram ∆x. ∆ν = 60626*10-34/...
Table-tennis ball has a mass 10 g and a speed of 90 m/s. If speed can be measured within an accuracy of 4% what will be the uncertainty in speed and position?
According to Heisenberg’s uncertainty principle: ∆x. ∆p ≥ h/4π Mass of the ball = 4 g Speed is = 90 m /s Uncertainity of speed, ∆v = 4/100 × 90 ∆v = 3.6 m/s ∆x = h/4πm∆v ∆x = 6.26 × 10-34 / 4 × 3.14...
What is the difference between the terms orbit and orbital?
Orbit represents a clear circular path for electrons to surround the nucleus. Represents the two-dimensional movement of electrons around the nucleus, the orbital is not that well defined because it...
Chlorophyll present in green leaves of plants absorbs light at 4.620 × 1014 Hz. Calculate the wavelength of radiation in nanometer. Which part of the electromagnetic spectrum does it belong to?
The relationship between the wavelength and the frequency: λ = c/ν c - Velocity of light v - Frequency of the radiation. λ = 3 x 108 ms-1 / 4.620 x 1014 Hz Hence, λ = 0.6494...
Out of electron and proton which one will have, a higher velocity to produce matter waves of the same wavelength? Explain it.
The electron which is a lighter particle will have the higher velocity and will also produce matter waves having the same wavelength. This is because, if the mass is less, then the velocity increases.
What is the experimental evidence in support of the idea that electronic energies in an atom are quantized?
The bright line spectrum shows that the atomic energy levels are measured. These lines are found to be the result of electronic transitions between energy and the atomic spectrum would have shown...
According to de Broglie, the matter should exhibit dual behavior that is both particle and wave-like properties. However, a cricket ball of mass 100 g does not move like a wave when it is thrown by a bowler at a speed of 100 km/h. Calculate the wavelength of the ball and explain why it does not show wave nature.
Calculation: Given, Mass, m = 100g / 0.1kg Velocity = 100km/h Velocity =100×1000 / 60×60 Velocity = 1000/36m/s λ =h/mν λ = 2.387 × 10-34 m
The Balmer series in the hydrogen spectrum corresponds to the transition from n1 = 2 to n2 = 3,4,………. This series lies in the visible region. Calculate the wavenumber of the line associated with the transition in Balmer series when the electron moves to n = 4 orbit. (RH= 109677 cm-1)
Calculation: According to Bohr’s model for the hydrogen atom; ν = RH(1/n12-1/ n22)cm-1 Given, n1 = 2 n2 = 4 H (Rydberg’s constant) = 109677 Wave number = 109677 ( ¼-1/16) Hence, Wave number =...
The electronic configuration of the valence shell of Cu is 3d10 4s1 and not 3d94s2. How is this configuration explained?
Great stability is established to the orbitals which are half or completely filled. In the given electronic configuration 3d104s1 of Copper (Cu), the stability is assured (d orbitals - filled, s...
Wavelengths of different radiations are given below :
λ(A) = 300 nm λ(B) = 300 μm λ(c) = 3 nm λ (D) 30 A° Arrange these radiations in the increasing order of their energies. Given, λ(A) = 300 nm λ(A) = 300 x 10-9 m λ(A) = 3 x 10 -7 m λ(B)...
An atom having atomic mass number 13 has 7 neutrons. What is the atomic number of the atom?
Calculation: Atomic mass number = number of protons + number of neutrons Number of protons = atomic mass number – number of neutrons. Hence, atomic number of an atom = 13 – 7 = 6.
Which of the following will not show deflection from the path on passing through an electric field? Proton, cathode rays, electron, neutron
Neutron shows no deflection from the path passing through the electric field. This is due to the neutrality of neutron particles. Therefore, it has no charge and is not affected by any electrical...
The arrangement of orbitals based on energy is based upon their (n+l ) value. Lower the value of (n+l ), lower is the energy. For orbitals having the same values of (n+l), the orbital with a lower value of n will have lower energy.
Based upon the above information, arrange the following orbitals in the increasing order of energy (a) 1s, 2s, 3s, 2p (b) 4s, 3s, 3p, 4d (c) 5p, 4d, 5d, 4f, 6s (d) 5f, 6d, 7s, 7p Based upon the...
Calculate the total number of angular nodes and radial nodes present in 3p orbital.
The region where the probability of finding the electrons is zero, it is considered as the nodes and is it present among the orbitals. Example: In the np orbitals, Nodes = n – l – 1 Nodes = 3 –1 – 1...
Which of the following orbitals are degenerate? 3dxy, 4dxy, 3dz2 , 3dyx, 4dyx, 4dzz
The electron energy in a multielectron atom, in contrast to the hydrogen atom, depends not only on its quantum number, but also on its azimuthal quantum number. The same electron shells and the same...
Nickel atom can lose two electrons to form Ni2+ ion. The atomic number of nickel is 28. From which orbital will nickel lose two electrons.
1 Ni atom = 28 electrons and its electronic configuration is 4s2 3d8 It turns to Ni2+ by losing 2 electrons and its electronic configuration becomes 4s0 3d8 According to the Aufbau principle, Ni...
Show the distribution of electrons in oxygen atom (atomic number 8) using orbital diagram.
Distribution of electrons in oxygen atom: 1s22s22p4
Arrange s, p and d sub-shells of a shell in the increasing order of effective nuclear charge (Zeff) experienced by the electron present in them.
Arrangement of the subshells: d<p<s The s-orbitals shield the electrons a lot more when compared to the p-orbitals from the nucleus.
Which of the following statements concerning the quantum numbers are correct?
(i) The angular quantum number determines the three-dimensional shape of the orbital. (ii) The principal quantum number determines the orientation and energy of the orbital. (iii) The magnetic...
In which of the following pairs, the ions are iso-electronic?
(i) Na+, Mg2+ (ii) Al3+, O– (iii) Na+, O2- (iv) N3-, Cl– Correct Answers: (i) Na+, Mg2+ (iii) Na+, O2- Explanation: Isoelectronic species are the atoms / ions that has the same number...
Which of the following sets of quantum numbers is correct? n l m n l m
(i) 1 1 +2 (ii) 2 1 +1 (iii) 3 2 –2 (iv) 3 4 –2 Correct Answers: (ii) 2 1 +1 (iii) 3 2 –2 Explanation: The correct sets of quantum numbers are, n = 2, l = 1, m = +1 n = 3, l = 2, m =...
Out of the following pairs of electrons, identify the pairs of electrons present in degenerate orbitals :
(i) (a) n = 3, l = 2, ml = –2, ms= − ½ (b) n = 3, l = 2, ml = –1, ms= − 1/2 (ii) (a) n = 3, l = 1, ml = 1, ms = + ½ (b) n = 3, l = 2, ml = 1, ms = +1/2 (iii) (a) n = 4, l = 1, ml = 1, ms = +...
Identify the pairs which are not of isotopes?
(i) 6X12, 6Y13 (ii) 17X35, 6Y37 (iii) 6X14, 7Y14 (iv) 4X8, 5Y8 Correct Answers: (iii) 6X14, 7Y14 (iv) 4X8, 5Y8 Explanation: Isotopes are the atoms having same atomic number but...
If travelling at the same speeds, which of the following matter waves have the shortest wavelength?
(i) Electron (ii) An alpha particle (He2+) (iii) Neutron (iv) Proton Correct Answer: (ii) An alpha particle (He2+) Explanation: According to de Broglie's equation, the alpha particles...
For the electrons of an oxygen atom, which of the following statements is correct?
(i) Zeff for an electron in a 2s orbital is the same as Zeff for an electron in a 2p orbital. (ii) An electron in the 2s orbital has the same energy as an electron in the 2p orbital. (iii) Zeff for...
The pair of ions having same electronic configuration is __________.
(i) Cr3+, Fe3+ (ii) Fe3+, Mn2+ (iii) Fe3+, Co3+ (iv) Sc3+, Cr3+ Correct Answer: (ii) Fe3+, Mn2+ Explanation: Fe - Z=26 : 3d64s2 Fe3+- 3d5 Mn - Z=25 : 3d54s2 Mn2+ : 3d5 Hence,...
Number of angular nodes for 4d orbital is __________.
(i) 4 (ii) 3 (iii) 2 (iv) 1 Correct Answer: (iii) 2 Explanation: The Number of angular nodes = l (azimuthal quantum number) Hence, the number of angular nodes for 4d orbital is...
Define the law of multiple proportions. Explain it with two examples. How does this law point to the existence of atoms?
When two elements combine to form two or more chemical compounds, then the mass of one of the compounds in a fixed mass of the other holds a simple measure of each other is the law of equality....
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction given below:
CaCO3 (s) + 2HCl (aq) → CaCl2(aq) + CO2(g) + H2O(l) What mass of CaCl2 will be formed when 250 mL of 0.76 M HCl reacts with 1000 g of CaCO3? Name the limiting reagent. Calculate the number of moles...
A vessel contains 1.6 g of dioxygen at STP (273.15K, 1 atm pressure). The gas is now transferred to another vessel at a constant temperature, where the pressure becomes half of the original pressure. Calculate: (i) the volume of the new vessel. (ii) a number of molecules of dioxygen.
(i) Calculation: Moles of oxygen = 1.6/32 Moles of oxygen = 0.05mol 1 mol of oxygen= 22.4L (at STP) Volume of Oxygen (V1) = 22.4 × 0.05 Volume of Oxygen (V1) = 1.12L V2 =? P1 = 1atm P2 = ½ P2 =...
Assertion (A): One atomic mass unit is defined as one-twelfth of the mass of one carbon-12 atom. Reason (R): Carbon-12 isotope is the most abundant isotope of carbon and has been chosen as the standard. (i) Both A and R are true and R is the correct explanation of A. (ii) Both A and R are true but R is not the correct explanation of A. (iii) A is true but R is false. (iv) Both A and R are false.
Correct Answer: (ii) Both A and R are true but R is not the correct explanation of A Explanation: The carbon 12 isotope defines the mass of atoms and molecules.
Assertion (A): The empirical mass of ethene is half of its molecular mass. Reason (R): The empirical formula represents the simplest whole-number the ratio of various atoms present in a compound. (i) Both A and R are true and R is the correct explanation of A. (ii) A is true but R is false. (iii) A is false but R is true. (iv) Both A and R are false.
Correct Answer: (i) Both A and R are true and R is the correct explanation of A Explanation: The empirical formula represents the simplest whole-number the ratio of various atoms present in a...
Match the following
Physical quantity Unit (i) Molarity (a) g mL–1 (ii) Mole fraction (b) mol (iii) Mole (c) Pascal (iv) Molality (d) Unitless (v) Pressure (e) mol L–1 (vi) Luminous intensity (e) mol L–1 (vii) Density...
Match the following
(i) 88 g of CO2 (a) 0.25 mol (ii) 6.022 ×1023 molecules of H2O (b) 2 mol (iii) 5.6 litres of O2 at STP (c) 1 mol (iv) 96 g of O2 (d) 6.022 × 1023 molecules (v) 1 mol of any gas (e) 3 mol ...
If 4 g of NaOH dissolves in 36 g of H2O, calculate the mole fraction of each component in the solution. Also, determine the molarity of the solution (specific gravity of solution is 1g mL–1).
Calculation: Mole fraction of H2O = Number of moles of H2O / Total number of moles (H2O +NaOH) Number of moles of H2O = 36/18 Number of moles of H2O =2 moles Number of moles of NaOH = 4/40 Number of...
The volume of a solution changes with change in temperature, then, will the molality of the solution be affected by temperature? Give a reason for your answer.
The Mass do not change when the temperature changes and so the molality of a solution do not change as well. Molality of a substance is defined as the number of mass of solute per mass of the...
Hydrogen gas is prepared in the laboratory by reacting dilute HCl with granulated zinc. Following reaction takes place.
Zn + 2HCl → ZnCl2 + H2 Calculate the volume of hydrogen gas liberated at STP when 32.65 g of zinc reacts with HCl. 1 mol of a gas occupies 22.7 L volume at STP; atomic mass of Zn = 65.3 u. ...
If two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in whole-number ratio. (a) Is this statement true? (b) If yes, according to which law? (c) Give one example related to this law.
(a) If two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in whole-number ratio and this statement is true....
Calculate the mass percent of calcium, phosphorus and oxygen in calcium phosphate Ca3(PO4)
Calculation: Molecular mass of Ca3(PO4) = (3 X 40) + (2 X 31) + (8 X 16) Molecular mass of Ca3(PO4) = 310 Mass percentage of Ca = \[\frac{3\times 40}{310\times 100}\] Mass percentage of Ca = 38.71%...
What is the difference between molality and molarity?
Molarity Molality Number of moles per volume of the solution in litres Number of mass of solute per mass of the solvent in liters Unit - M Unit - m
What is the symbol for the SI unit of a mole? How is the mole defined?
Mole is the amount of substance containing more entities because there are atoms in 12 g of carbon. SI unit symbol – mol
How many significant figures should be present in the answer of the following calculations? 2.5×1.25×3.5/ 2.01
Number Significant figures 2.5 2 1.25 3 3.5 2 2.01 3 In the given calculation, involving both the multiplication and division, the significant figures present is 2. Hence, the result cannot have...
What will be the mass of one atom of C-12 in grams?
1 mole of carbon atom = 12g Therefore, Mass of one atom of C-12 in grams= 1.99 × 1023 grams.
One of the statements of Dalton’s atomic theory is given below: “Compounds are formed when atoms of different elements combine in a fixed ratio” Which of the following laws is not related to this statement? (i) Law of conservation of mass (ii) Law of definite proportions (iii) Law of multiple proportions (iv) Avogadro’s law
Correct Answers: (i) Law of conservation of mass; (iv) Avogadro's law Explanation: According to the Dalton's atomic theory, The Chemical compounds are formed when atoms of various elements join in a...
Which of the following solutions have the same concentration?
(i) 20 g of NaOH in 200 mL of solution (ii) 0.5 mol of KCl in 200 mL of solution (iii) 40 g of NaOH in 100 mL of solution (iv) 20 g of KOH in 200 mL of solution Answer: Correct Answers: (i)...
Which of the following pairs have the same number of atoms?
(i) 16 g of O2(g) and 4 g of H2(g) (ii) 16 g of O2 and 44 g of CO2 (iii) 28 g of N2 and 32 g of O2 (iv) 12 g of C(s) and 23 g of Na(s) Answer: Correct Answers: (iii) 28 g of N2 and 32 g of...
Sulphuric acid reacts with sodium hydroxide as follows:
H2SO4 + 2NaOH → Na2SO4+ 2H2O When 1L of 0.1M sulphuric acid solution is allowed to react with 1L of 0.1M sodium hydroxide solution, the amount of sodium sulphate formed and its molarity in the...
One mole of oxygen gas at STP is equal to _______.
(i) 6.022 × 1023 molecules of oxygen (ii) 6.022 × 1023 atoms of oxygen (iii) 16 g of oxygen (iv) 32 g of oxygen Answer: Correct Answers: (i) 6.022 × 1023 molecules of oxygen; (iv) 32 g of...
Which of the following statements indicates that the law of multiple proportions is being followed.
(i) Sample of carbon dioxide taken from any source will always have carbon and oxygen in the ratio 1:2. (ii) Carbon forms two oxides namely CO2 and CO, where masses of oxygen which combine with a...
Which of the following reactions is not correct according to the law of conservation of mass.
(i) 2Mg(s) + O2(g) →2MgO(s) (ii) C3H8(g) + O2(g) → CO2(g) + H2O(g) (iii) P4(s) + 5O2(g) → P4O10(s) (iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O (g) Answer: Correct Answer: (ii) C3H8(g) + O2(g) →...
Which of the following statements is correct about the reaction given below: 4Fe(s) + 3O2(g) → 2Fe2O3(g) (i) The total mass of iron and oxygen in reactants = total mass of iron and oxygen in product therefore it follows the law of conservation of mass. (ii) The total mass of reactants = total mass of product; therefore, the law of multiple proportions is followed. (iii) Amount of Fe2O3 can be increased by taking any one of the reactants (iron or oxygen) in excess. (iv) Amount of Fe2O3 produced will decrease if the amount of any one of the reactants (iron or oxygen) is taken in excess.
Correct Answer: (i) The total mass of iron and oxygen in reactants = total mass of iron and oxygen in product therefore it follows the law of conservation of mass. Explanation: From the reaction,...
Which of the following statements about a compound is incorrect? (i) A molecule of a compound has atoms of different elements. (ii) A compound cannot be separated into its constituent elements by physical methods of separation. (iii) A compound retains the physical properties of its constituent elements. (iv) The ratio of atoms of different elements in a compound is fixed.
Correct Answer: (iii) A compound retains the physical properties of its constituent elements Explanation: Molecule of a compound is made up of atoms of various elements which cannot be separated...
If the density of a solution is 3.12 g mL-1, the mass of 1.5 mL solution in significant figures is _______. (i) 4.7g (ii) 4680 × 10 -3g (iii) 4.680g (iv) 46.80g
Correct Answer: (i) 4.7g Explanation: Given, Density of solution = 3.12 g mL-1 Volume of solution = 1.5 mL Formula for Mass = Volume × Density Hence, Mass = 4.7 g
The empirical formula and molecular mass of a compound are CH2O and 180 g respectively. What will be the molecular formula of the compound? (i) C9H18O9 (ii) CH2O (iii) C6H12O6 (iv) C2H4O2
Correct Answer: (iii) C6H12O6 Explanation: Given, Molar mass of Carbon=12 Molar mass of Hydrogen=1 Molar mass of Oxygen=16 So, The molecular weight of compound is 6 and so the molecular formula of...
What is the mass per cent of carbon in carbon dioxide? (i) 0.034% (ii) 27.27% (iii) 3.4% (iv) 28.7%
Correct Answer: (ii) 27.27% Explanation: Carbon dioxide is a gas with a density of about 53% higher than that of dry air. Carbon dioxide molecules consist of a double carbon atom combined with two...
One mole of any substance contains 6.022 × 1023 atoms/molecules. Number of molecules of H2SO4 present in 100 mL of 0.02M H2SO4 solution is ______. (i) 12.044 × 1020 molecules (ii) 6.022 × 1023 molecules (iii) 1 × 1023 molecules (iv) 12.044 × 1023molecules
Correct Answer: (i) 12.044 × 1020 molecules Explanation: Moles of H2SO4= Molarity of H2SO4×Volume of solution (L) Hence, the number of molecules of H2SO4 present in 100 mL of 0.02M H2SO4 solution...
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3. The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of the above compounds is : (i) HF > H2O > NH3 (ii) H2O > HF > NH3 (iii) NH3 > HF > H2O (iv) NH3 > H2O > HF
Solution: Option (ii) is the answer.
The types of hybrid orbitals of nitrogen in NO2+, NO3- and NH4+respectively are expected to be
(i) sp, sp3 and sp2
(ii) sp, sp2 and sp3
(iii) sp2, sp and sp3
(iv) sp2, sp3 and sp
Solution: Option (ii) is the answer. The hybridisation of each molecule gives us an idea about the hybrid orbitals.
Assertion (A): Electron gain enthalpy becomes less negative as we go down a group.
Reason (R): Size of the atom increases on going down the group and the added electron would be farther from the nucleus.
(a) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
(b) Assertion and reason both are correct statements and reason is correct explanation of assertion.
(c) Assertion and reason both are wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(b) As one moves down the group, the electron gain enthalpy decreases because the atomic size grows and the new electron is further away from the nucleus.
Assertion (A): Boron has a smaller first ionization enthalpy than beryllium. Reason (R): The penetration of a 2s electron to the nucleus is more than the 2p electron, hence, 2p electron is more shielded by the inner core of electrons that the 2s electrons.
(a) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
(b) Assertion is correct statement but reason is wrong statement.
(c) Assertion and reason both are correct statements and reason is correct explanation of assertion.
(d) Assertion and reason both are wrong statement.
(c) Because beryllium (1s2 2s2) has a fully filled, boron (1s2 2s2 2p1) has a lower initial ionisation enthalpy than beryllium (1s2 2s2). s-subshell. When compared to 2s-electrons, 2s-electrons are...
Assertion and Reason Type Questions
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given.
Choose the correct option out of the choices given below each question.
Assertion (A): Generally, ionization enthalpy increases from left to right in a period.
Reason (R): When successive electrons are added to the orbitals in the same principal quantum level, the shielding effect of inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus.
(a) Assertion is correct statement and reason is wrong statement.
(b) Assertion and reason both are correct statements and reason is correct explanation of assertion.
(c) Assertion and reason both are wrong statements.
(d) Assertion is wrong statement and reason is correct statement.
(b) As atomic size decreases, the ionization enthalpy increases from left to right over time. The effective nuclear charge of the electrons in the subshell is about the same.
Define ionisation enthalpy. Discuss the factors affecting ionisation enthalpy of the elements and its trends in the periodic table.
The energy required by an isolated, gaseous atom in its ground state to remove an electron is known as ionization enthalpy. The valence electrons are shielded by the inner electrons, which reduces...
Discuss the factors affecting electron gain enthalpy and the trend in its variation in the periodic table.
The factors affecting electron gain enthalpy and the trend in its variation in the periodic table are: Atomic size - As the distance between the nucleus and the outermost shell rises, the tendency...
Among alkali metals which element do you expect to be least electronegative and why?
Because electronegativity decreases as we move down the group, caesium is the least electronegative alkali metal. Cesium is a group 1 element with the biggest size due to a drop in the effective...
The radius of Na+ cation is less than that of Na atom. Give reason.
The sodium atom loses one electron to form a sodium cation, and the effective nuclear charge on the ion increases on the left electrons after the cation is formed, resulting in a decrease in radius.
How does the metallic and non-metallic character vary on moving from left to right in a period?
Metallic character reduces as we move from left to right across the period, whereas non-metallic character increases as ionization enthalpy and electron gain enthalpy increase across the period
Explain the following:
(a) Electronegativity of elements increases on moving from left to right in the periodic table.
(b) Ionisation enthalpy decrease in a group from top to bottom?
(a) The size of the atoms reduces as we move from left to right in a period due to an increase in the effective nuclear charges on the outermost electron. As a result, as you move from left to right...
Explain the deviation in ionisation enthalpy of some elements from the general trend by using the given figure:
Solution: Ionization enthalpy of elements varies through period and group. As we move from left to right in a period, the ionization enthalpy increases and lowers when we move down a group....
Arrange the elements N, P, O and S in the order of-
(i) increasing first ionisation enthalpy.
(ii) increasing non-metallic character.
Give the reason for the arrangement assigned.
(i) The ascending order of the initial ionization enthalpy is S< P< O< N. The ionization enthalpy drops as we move down the group and increases as we move along the period, but in the case...
What do you understand by exothermic reaction and endothermic reaction? Give one example of each type.
Exothermic reaction: An exothermic reaction is a reaction in which heat is released during the reaction. For instance, Cao + CO2→ CaCO3 ΔH=-178kJmol-1 Endothermic reaction: An endothermic reaction...
How would you explain the fact that first ionisation enthalpy of sodium is lower than that of magnesium but its second ionisation enthalpy is higher than that of magnesium?
When sodium loses an electron from its outermost shell, it achieves a stable state. As a result, its initial ionization enthalpy is lower than that of magnesium. However, in the case of second...
p-Block elements form acidic, basic and amphoteric oxides. Explain each property by giving two examples and also write the reactions of these oxides with water.
ACIDIC OXIDES Acidic oxides are oxides that react with water to produce acids. SO2, B2O3 are acidic oxides and p block elements. The chemical equation for the reaction of B2O3 with water:- B2O3 +3...
The first member of each group of representative elements (i.e., s and p-block elements) shows anomalous behaviour. Illustrate with two examples.
Examples include lithium and beryllium. The initial group element is Li. It has variety of characteristics and forms, including covalent compounds and nitrides. The second group's initial element is...
Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
The ionization enthalpy of oxygen is lower than that of nitrogen because when one electron is removed from oxygen, it easily donates it to achieve half-filled stability, whereas removing one...
Illustrate by taking examples of transition elements and non-transition elements that oxidation states of elements are largely based on electronic configuration.
Ti has an atomic number of 22 and an electronic configuration of [Ar]3d24s2. It may be found in various compounds with three different oxidation states of +2,+3, and +4 such as TiO2(+4), Ti2O3(+3),...
Choose the correct order of atomic radii of fluorine and neon (in pm) out of the options given below and justify your answer.
(i) 72, 160
(ii) 160, 160
(iii) 72, 72
(iv) 160, 72
(i) 72, 160 Neon has van der Waal's radii and fluorine has covalent radii. The covalent radius is always less than van der Waal's radius, Fluorine has a radius of 72pm while Neon has a radius of...
Write four characteristic properties of p-block elements.
They have a wide range of oxidation states. The reducing character increases as we move down the group, while the oxidizing character increases across the period. The ionization enthalpy of these...
Among the elements B, Al, C and Si,
(i) which element has the highest first ionisation enthalpy?
(ii) which element has the most metallic character? Justify your answer in each case.
(i) The ionization enthalpy of carbon is the highest. It rises from left to right along the period and then decreases as we move down the group. (ii) The most metallic element is aluminium. The...
Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of Does the following have the highest dipole moment? (i) CO2 (ii) HI (iii) H2O (iv) SO2
H2O has a high dipole moment because Oxygen is highly electronegative. This attracts the electron from Hydrogen towards it with greater charge. Therefore, H2O...
The statement that is not correct for periodic classification of elements is:
(i) The properties of elements are periodic function of their atomic numbers.
(ii) Non-metallic elements are less in number than metallic elements.
(iii) For transition elements, the 3d-orbitals are filled with electrons after3p-orbitals and before 4s-orbitals.
(iv) The first ionisation enthalpies of elements generally increase withincrease in atomic number as we go along a period.
Option (iii) is the answer. The Aufbau principle describes how electrons first fill low-energy orbitals (near to the nucleus) before moving on to higher-energy orbitals. They fill the orbitals...
Ionisation enthalpies of elements of the second period are given below: Ionisation enthalpy/ kcal mol–1: 520, 899, 801, 1086, 1402, 1314, 1681, 2080. Match the correct enthalpy with the elements and complete the graph given in Fig. 3.1. Also, write symbols of elements with their atomic number.
Solution: N has a higher first ionisation enthalpy than O, despite the fact that O has a higher nuclear charge. This is because the electron in N must be removed from a more stable, exactly...
Identify the group and valency of the element having atomic number 119. Also, predict the outermost electronic configuration and write the general formula of its oxide.
The modern periodic table has 118 elements divided into seven periods. As a result, the element with atomic number 119 will be in the 8th period of the first group and will have the electronic...
All transition elements are d-block elements, but all d-block elements do not transition elements. Explain.
D block elements are those that have their outermost shell filled with d electrons. Because incompletely filled d orbitals are crucial for elements like calcium and zinc, all d block elements are...
Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine.
Fluorine has a lower size than chlorine, which means there is less attraction outside the shell to gain an electron. In addition, inter-electronic repulsions exist in the 2p orbitals, resulting in a...
An element belongs to the 3rd period and group-13 of the periodic table. Which of the following properties will be shown by the element?
(i) Good conductor of electricity
(ii) Liquid, metallic
(iii) Solid, metallic
(iv) Solid, non-metallic
Option (i) and (iii) are the answers. The element belonging to 3rd period and 13th group is aluminium which is a metal. Hence, it is solid, metallic and good conductor of electricity.
Ionic radii vary in
(i) inverse proportion to the effective nuclear charge.
(ii) inverse proportion to the square of effective nuclear charge.
(iii) direct proportion to the screening effect.
(iv) direct proportion to the square of screening effect.
Option (i) and (iii) are the answers. Ionic radii decreases as the effective nuclear charge increases due to inverse proportional relation. Also, ionic radii increases as the screening effect...
Which of the following have no unit?
(i) Electronegativity
(ii) Electron gain enthalpy
(iii) Ionisation enthalpy
(iv) Metallic character
Option (i) and (iv) are the answers. Electron gain enthalpy and ionization enthalpy have units of enthalpy.
In which of the following options order of arrangement does not agree with
the variation of property indicated against it?
(i) Al3+ < Mg2+ < Na+ < F– (increasing ionic size)
(ii) B < C < N < O (increasing first ionisation enthalpy)
(iii) I < Br < Cl < F (increasing electron gain enthalpy)
(iv) Li < Na < K < Rb (increasing metallic radius)
Option (ii) and (iiii) are the answers. For increasing first ionization enthalpy, the order should be: B < C < O < N For increasing electron gain enthalpy, the order should be: I < Br...
Which of the following sets contain only isoelectronic ions?
![Rendered by QuickLaTeX.com \[\left( \mathbf{i} \right)\text{ }\mathbf{Z}{{\mathbf{n}}^{\mathbf{2}+}},\text{ }\mathbf{C}{{\mathbf{a}}^{\mathbf{2}+}},\text{ }\mathbf{G}{{\mathbf{a}}^{\mathbf{3}+}},\text{ }\mathbf{A}{{\mathbf{l}}^{\mathbf{3}+}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f9b656c6f512de0f78f5c9e95ea9e1bc_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }{{\mathbf{K}}^{+}},\text{ }\mathbf{C}{{\mathbf{a}}^{\mathbf{2}+}},\text{ }\mathbf{S}{{\mathbf{c}}^{\mathbf{3}+}},\text{ }\mathbf{C}{{\mathbf{l}}^{}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-02a8f827fa33900690bdb99cc37e81aa_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }{{\mathbf{P}}^{\mathbf{3}-}},\text{ }{{\mathbf{S}}^{\mathbf{2}-}},\text{ }\mathbf{C}{{\mathbf{l}}^{}},\text{ }{{\mathbf{K}}^{+}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-9e135ccca95537425808d01a2026aa19_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iv} \right)\text{ }\mathbf{Ti}{{~}^{\mathbf{4}+}},\text{ }\mathbf{Ar},\text{ }\mathbf{C}{{\mathbf{r}}^{\mathbf{3}+}},\text{ }{{\mathbf{V}}^{\mathbf{5}+}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-31b52550d9bb732119e63c874ec47f83_l3.png)
Option (ii) and (iii) are the answers. (i) Zn2+ (30 – 2 = 28), Ca2+ (20 – 2 = 18), Ga3+ (31-3= 28), Al3+ (13 – 3 = 10) are not isoelectronic. (ii) K+ (19 – 1 = 18), Ca2+ (20 – 2 = 18), Sc3+ (21 – 3...
Which of the following statements are correct?
(i) Helium has the highest first ionisation enthalpy in the periodic table.
(ii) Chlorine has less negative electron gain enthalpy than fluorine.
(iii) Mercury and bromine are liquids at room temperature.
(iv) In any period, the atomic radius of alkali metal is the highest.
Option (i), (iii) and (iv) are the answers. Because of its larger size and lower electronic repulsion, chlorine has a higher negative electron gain enthalpy than fluorine.
Which of the following elements will gain one electron more readily in comparison to other elements of their group?
(i) S (g)
(ii) Na (g)
(iii) O (g)
(iv) Cl (g)
Option (i) and (iv) are the answers. Chlorine has the strongest tendency to gain an electron, as well as a high electron gain enthalpy (-ve). Group 16 includes O and S, however S has a higher...
Which of the following sequences contain atomic numbers of only representative elements?
(i) 3, 33, 53, 87
(ii) 2, 10, 22, 36
(iii) 7, 17, 25, 37, 48
(iv) 9, 35, 51, 88
Option (i) and (iv) are the answers. Representative elements are elements from the 5 and p-blocks. Transition elements belong to the f-block (Z=21–30; 39–48; 57 and 72–80; 89 and 104–112), while...
Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of the spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
Option (i) and (iii) are the answers. Ionization enthalpies are low in group 1 (alkali metals) and group 2 (alkaline earth metals). As a result, they give flame colour.
Which of the following elements can show covalency greater than 4?
(i) Be
(ii) P
(iii) S
(iv) B
Option (ii) and (iii) are the answers. Because P and S have d-orbitals in their valence shells, they can hold more than eight electrons in their valence shells. As a result, they have a covalency of...
Electronic configurations of four elements A, B, C and D are given below :
![Rendered by QuickLaTeX.com \[\left( \mathbf{A} \right)\text{ }\mathbf{1}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{p}}^{\mathbf{6}}}~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-4e5bb330482f0744293b4adfc531e398_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{B} \right)\text{ }\mathbf{1}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{p}}^{\mathbf{4}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-d6053c2db0981662ae5726905c36efc8_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{C} \right)\text{ }\mathbf{1}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{p}}^{\mathbf{6}}}~\mathbf{3}{{\mathbf{s}}^{\mathbf{1}}}~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a76e95b592ec114208946333898c356f_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{D} \right)\text{ }\mathbf{1}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{s}}^{\mathbf{2}}}~\mathbf{2}{{\mathbf{p}}^{\mathbf{5}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-39a728458277f2c0c56b6f3caacd43ef_l3.png)
Which of the following is the correct order of increasing tendency to gain electron :
(i) A < C < B < D
(ii) A < B < C < D
(iii) D < B < C < A
(iv) D < A < B < C
Option (i) is the answer. (a) A – Is2 2s2 2p6 – Noble gas configuration B -1s2 2s2 2p4 – 2 electrons short of stable configuration C – 1s2 2s2 2p6 3.?1 – Requires one electron to complete 5-orbital...
Comprehension given below is followed by some multiple-choice questions.
Each question has one correct option. Choose the correct option.
In the modern periodic table, elements are arranged in order of increasingatomic numbers which are related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic the table has been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Aufbau principle, the seven periods (1 to 7) have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
(i) The element with atomic number 57 belongs to
(a) s-block
(b) p-block
(c) d-block
(d) f-block
(ii) The last element of the p-block in 6th period is represented by the outermost electronic configuration.
(a) 7s2 7p6
(b) 5f 14 6d10 7s 2 7p 0
(c) 4f 14 5d10 6s2 6p6
(d) 4f 14 5d10 6s 2 6p 4
(iii) Which of the elements whose atomic numbers are given below, cannot be accommodated in the present set up of the long form of the periodic table?
(a) 107
(b) 118
(c) 126
(d) 102
(e) The electronic configuration of the element which is just above the element with atomic number 43 in the same group is ________.
(a) 1s2 2s2 2p6 3s2 3p6 3d5 4s2
(b) 1s2 2s2 2p6 3s2 3p6 3d5 4s3 4p6
(c) 1s2 2s2 2p6 3s2 3p6 3d6 4s2
(d) 1s2 2s2 2p6 3s2 3p6 3d7 4s2
(v) The elements with atomic numbers 35, 53 and 85 are all ________.
(a) noble gases
(b) halogens
(c) heavy metals
(d) light metals
The formation of the oxide ion, O2- (g), from oxygen atom requires first an exothermic and then an endothermic step as shown below:
![Rendered by QuickLaTeX.com \[\mathbf{O}\text{ }\left( \mathbf{g} \right)\text{ }+\text{ }\mathbf{e}\text{ }\to \text{ }{{\mathbf{O}}^{-}}\left( \mathbf{g} \right)\text{ };\text{ };\text{ }\text{ }\mathbf{HV}\text{ }=\text{ }\text{ }\mathbf{141}\text{ }\mathbf{kJ}\text{ }\mathbf{mol}\mathbf{1}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-8a59c39962cc77d1f2cc97ee1ec0e6bf_l3.png)
![Rendered by QuickLaTeX.com \[{{\mathbf{O}}^{-}}\left( \mathbf{g} \right)\text{ }+\text{ }\mathbf{e}\text{ }\to \text{ }{{\mathbf{O}}^{\mathbf{2}-}}\left( \mathbf{g} \right)\text{ };\text{ }\text{ }\mathbf{HV}\text{ }=\text{ }+\text{ }\mathbf{780}\text{ }\mathbf{kJ}\text{ }\mathbf{mol}\mathbf{1}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-105b68e8c79fc1248ada69c0cf9a98de_l3.png)
Thus the process of formation of O2– in the gas phase is unfavourable even though O2- is isoelectronic with neon. It is due to the fact that
(i) oxygen is more electronegative.
(ii) addition of electron in oxygen results in larger size of the ion.
(iii) electron repulsion outweighs the stability gained by achieving a noble gas configuration.
(iv) O- ion has a comparatively smaller size than an oxygen atom.
Option (iii) is the answer. This is due to the fact that when an electron is introduced to a negatively charged ion, it is repelled rather than attracted. As a result, the addition of the second...
Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs. (i) [NF3 and BF3] (ii) [BF4- and NH4+] (iii) [BCl3 and BrCl3] (iv) [NH3 and NO3-]
From a structural standpoint, we can see that, NF3 is pyramidal whereas BF3 is planar triangular. BF4- and NH4+ ions are tetrahedral in structure. BCl3 is triangular planar and BrCl3 is...
Which of the following is the correct order of the size of the given species:
![Rendered by QuickLaTeX.com \[~\left( \mathbf{i} \right)\text{ }\mathbf{I}\text{ }>\text{ }{{\mathbf{I}}^{-}}>\text{ }{{\mathbf{I}}^{+}}^{{}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7a8b80587c7e4bf72c9cd196715177bc_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }\mathbf{I}+>\text{ }{{\mathbf{I}}^{-}}>\text{ }\mathbf{I}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a7464c368804f0a28cde1894bd32bb60_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }\mathbf{I}\text{ }>\text{ }{{\mathbf{I}}^{+}}>\text{ }{{\mathbf{I}}^{-}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-61edf3a246a74100367d9431971a553f_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iv} \right)\text{ }{{\mathbf{I}}^{-}}>\text{ }\mathbf{I}\text{ }>\text{ }{{\mathbf{I}}^{+}}^{{}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7de79f7e5e8187314bd7962083407d61_l3.png)
Option (iv) is the answer. The size of an anion, cation, and neutral species for a given element is in this order: anion>element>cation. Because of its higher z-effective, cation has the...
The elements in which electrons are progressively filled in 4f-orbital are called
(i) actinoids
(ii) transition elements
(iii) lanthanoids
(iv) halogens
Option (iii) is the answer. In lanthanoids, the 4f orbital is gradually filled with electrons. Lanthanoids have a broad electrical configuration. [Xe]4f 1-145d0-16s2.
The period number in the long form of the periodic table is equal to
(i) magnetic quantum number of any element of the period.
(ii) an atomic number of any element of the period.
(iii) maximum Principal quantum number of any element of the period.
(iv) maximum Azimuthal quantum number of any element of the period.
Option (iii) is the answer. Period number = maximum n of any element where 'n' stands for the principle quantum number. It determines the element's period number. Mg, for example, has a maximum main...
The first ionisation enthalpies of Na, Mg, Al and Si are in the order:
(i) Na < Mg > Al < Si
(ii) Na > Mg > Al > Si
(iii) Na < Mg < Al < Si
(iv) Na > Mg > Al < Si
Option (i) is the answer. Ionization enthalpy is the enthalpy change associated with the loss of the first electron from an isolated gaseous atom in its ground state. As we move across the period,...
The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is:
(i) s > p > d > f
(ii) f > d > p > s
(iii) p < d < s > f
(iv) f > p > s > d
Option (i) is the answer. In every atom with more than one electron shell, this effect, known as the screening effect, describes the decrease in attraction between an electron and the nucleus. The...
Which of the following is not an actinoid?
(i) Curium (Z = 96)
(ii) Californium (Z = 98)
(iii) Uranium (Z = 92)
(iv) Terbium (Z = 65)
Option (iv) is the answer. Any group of 15 elements in the periodic table, ranging from actinium to lawrencium (atomic numbers 89–103), is known as an actinoid element. As evident from the...
Consider the isoelectronic species, Na+, Mg2+, F–and O2–. The correct order of increasing length of their radii is _________.
![Rendered by QuickLaTeX.com \[\left( \mathbf{i} \right)\text{ }{{\mathbf{F}}^{}}~<\text{ }{{\mathbf{O}}^{\mathbf{2}-}}~<\text{ }\mathbf{M}{{\mathbf{g}}^{\mathbf{2}+}}~<\text{ }\mathbf{N}{{\mathbf{a}}^{+}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-acaf0dffafbb532357bb7c961ba42536_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }\mathbf{M}{{\mathbf{g}}^{\mathbf{2}+}}~<\text{ }\mathbf{N}{{\mathbf{a}}^{+}}<\text{ }{{\mathbf{F}}^{}}<\text{ }{{\mathbf{O}}^{\mathbf{2}-}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-59b8fd0e0bc02f09a0dce6105e1a3ee9_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }{{\mathbf{O}}^{\mathbf{2}-}}<\text{ }{{\mathbf{F}}^{}}~<\text{ }\mathbf{N}{{\mathbf{a}}^{+}}<\text{ }\mathbf{M}{{\mathbf{g}}^{\mathbf{2}+}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-70ee46859ecff0f07d87ddf80025e958_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iv} \right)\text{ }{{\mathbf{O}}^{\mathbf{2}-}}~<\text{ }{{\mathbf{F}}^{}}~<\text{ }\mathbf{M}{{\mathbf{g}}^{\mathbf{2}+}}~<\text{ }\mathbf{N}{{\mathbf{a}}^{+}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-dd8b58c28bec5d6d898ba3debf2d258c_l3.png)
Option (ii) is the answer. Radius of elements increase as we move from left to right in a period. These are isoelectronic species having same number of electrons. As we move from left to right, from...
Give the disproportionation reaction of H3PO3.
When we heat orthophosphorus acid (H3PO3), it undergoes disproportionation reaction to yield orthophosphoric acid (H3PO4) and phosphine (PH3). The oxidation states of phosphorous atom in...
Write the main differences between the properties of white phosphorus and red phosphorus.
Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
Nitrogen because of its small size has a capacity to form pπ−pπ multiple bonds with itself.Thus, nitrogen forms a very stable diatomic molecule, N2. On moving down a group, the tendency...
Explain why NH3 is basic while BiH3 is only feebly basic.
NH3 is distinctly basic while BiH3 is feebly basic as nitrogen has a smaller size due to which the lone pair of electrons are concentrated in a small area. This means that the charge density per...
Why does R3P=O exist but R3N=O does not (R = alkyl group)?
Nitrogen, N (unlike P) does not have the d-orbital. This restricts nitrogen to expand its coordination number beyond four. Hence, R3N=O does not exist and it cannot accommodate more electrons due...
The HNH angle value is higher than HPH, HAsH and HSbH angles. Why? [Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s−p bonding between hydrogen and other elements of the group.
The H-M-H bond angles for the hydrides of group-15 elements are as follows: NH3 = 107o PH3 = 92o AsH3 = 91o SbH3 = 90oThe above trend in the H−M−H bond angle can be explained on the basis of the...
Give the resonating structures of NO2 and N2O5.
The resonating structures of the given compounds are as follows: NO2 : N2O5 :
Illustrate how copper metal can give different products on reaction with HNO3.
Concentrated nitric acid acts as a strong oxidizing agent. It is used for the oxidation of most metals.The products of oxidation depend on certain parameters such as temperature, concentration of...
Define the following as related to proteins (i) Primary structure (ii) Peptide linkage (iii) Denaturation.
(i) Primary structure When we discuss the primary structure of a protein, we refer to the exact sequence in which the amino acids are present. For example, the sequence of amino acid linkages in a...
