Frequency of radiation $(\nu)$, $\nu=\frac{1}{2.0 \times 10^{-9} s}$ $\nu=5.0 \times 10^{8} s^{-1}$ Energy $(E)$ of source $=$ Nhv Where, $N$ is the no. photons emitted $\mathrm{h}$ is Planck's...
Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nanosecond range. If the radiation source has the duration of
Arrange the following type of radiations in increasing order of frequency: (a) radiation from microwave oven (b) amber light from traffic signal (c) radiation from FM radio (d) cosmic rays from outer space and (e) X-rays.
The following is the frequency order in ascending order: Radiation from FM radio < amber light < radiation from microwave oven < X- rays < cosmic rays The following is the increasing...
Explain, giving reasons, which of the following sets of quantum numbers are not possible.
a) 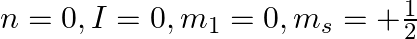
b) 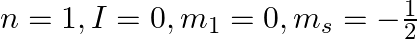
c) 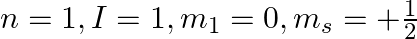
d) 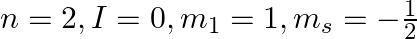
e) 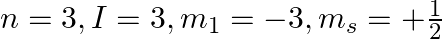
f) 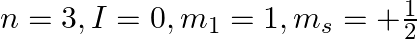
a) This is not possible. The number n cannot be zero. (b) Possible. (c) This is not possible. The value of l can't be the same as the value of n. (d) This is not possible. Because mt can't be 1 when...
What is the lowest value of n that allows g orbitals to exist?
For g-orbitals, l = 4. The possible values of ‘l’ range from 0 to (n-1),. For any given value of ‘n’, Hence, least value of n = 5, l = 4 (g orbital),
(II) What are the atomic numbers of elements whose outermost electrons are represented by (a) 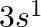 (b)
(b) 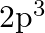 and (c)
and (c) 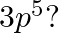
(II) (a) $3 \mathrm{~s}^{1}$ Complete electronic configuration: $1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}^{6} 3 \mathrm{~s}^{1}$ Total no. electrons in the atom $=2+2+6+1=11 \quad...
The electron energy in hydrogen atom is given by 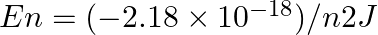 . Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength of light in cm that can be used to cause this transition?
. Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength of light in cm that can be used to cause this transition?
Required energy for the ionization from $\mathrm{n}=2$ is: $ \begin{array}{l} \Delta E=E_{\infty}-E_{2} \\ =\left[\left(\frac{-\left(2.18 \times...
What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the light emitted when the electron returns to the ground state? The ground state electron energy is 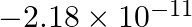 ergs. The ground-state electron energy is
ergs. The ground-state electron energy is 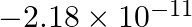 ergs.
ergs.
$ E_{5}=\frac{-\left(2.18 \times 10^{-18}\right) Z^{2}}{(n)^{2}} $ Where, $Z$ denotes the atom's atomic number Ground state energy $=-2.18 \times 10^{-11}$ ergs $=-2.18 \times 10^{-11} \times...
Calculate the wavenumber for the longest wavelength transition in the Balmer series of atomic hydrogen.
The Balmer series of the hydrogen emission spectrum, ni = 2. Hence, wavenumber expression ν is: $ \bar{\nu}=\left[\frac{1}{(2)^{2}}-\frac{1}{n_{f}^{2}}\right]\left(1.097 \times 10^{7}...
The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. If any of these combination(s) has/have the same energy lists: n = 4, l = 2, ml = –2 , ms = –1/2 n = 3, l = 2, ml= 1 , ms = +1/2 n = 4, l = 1, ml = 0 , ms = +1/2 n = 3, l = 2, ml = –2 , ms = –1/2 n = 3, l = 1, ml = –1 , ms= +1/2 n = 4, l = 1, ml = 0 , ms = +1/2
The 4d, 3d, 4p, 3d, 3p, and 4p orbitals are home to electrons 1, 2, 3, 4, 5, and 6. (respectively). Ranking these orbitals in the increasing order of energies: (3p) < (3d) < (4p) < (4d).
The unpaired electrons in Al and Si are present in 3p orbital. Which electrons will experience more effective nuclear charge from the nucleus?
The net positive charge acting on an electron in an atom's orbital with more than one electron is known as the nuclear charge. The nuclear charge increases as the atomic number increases. Silicon...
Calculate the wavelength, frequency and wavenumber of a light wave whose period is 2.0 × 10–10 s.
Frequency of the light wave $\nu$ = $\frac{1}{Period} \frac{1}{Period}$ $=\frac{1}{2.0\times 10^{-10}\, s} =5.0\times 10^{9}\, s^{-1 }$ Wavelength of the light wave$\lambda=c\nu$ Where, c denotes...
How many neutrons and protons are there in the following nuclei? 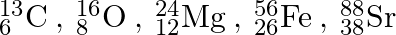
\({}_{6}^{13}C\): Mass number of carbon-13 = 13 Atomic number of carbon = Number of protons in one carbon atom = 6 Therfore, total number of neutrons in 1 carbon atom = Mass number – Atomic number =...
(i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons.
1 electron weighs 9.109*10-31 kg. Therefore, number of electrons that weigh 1 g (10-3 kg) = 1.098*1027 electrons (ii) Mass of one mole of electrons = NA* mass of one electron =...
Increase in kinetic energy can overcome intermolecular forces of attraction. How will the viscosity of liquid be affected by the increase in temperature? (i) Increase (ii) No effect (iii) Decrease (iv) No regular pattern will be followed
The correct option is (iii) Decrease.
What is the SI unit of viscosity coefficient (η)? (i) Pascal (ii) Nsm–2 (iii) km–2 s (iv) N m–2
The correct option is (ii) Nsm–2
The interaction energy of the London force is inversely proportional to the sixth power of the distance between two interacting particles but their magnitude depends upon (i) charge of interacting particles (ii) mass of interacting particles (iii) polarisability of interacting particles (iv) strength of permanent dipoles in the particles.
The correct option is (iii) polarisability of interacting particles.
How many neutrons and protons are there in the following nuclei?
Ans: 24 is the mass number. The number of protons in an atom equals the number of atoms in the atom. Mass number – Atomic number = 24 – 12 = 12 neutrons Numerical value of mass nubmer = 56 Number...
Assertion (A): The black body is an ideal body that emits and absorbs radiations of all frequencies. Reason (R): The frequency of radiation emitted by a body goes from a lower frequency to higher frequency with an increase in temperature.
(i) Both A and R are true and R is the correct explanation of A. (ii) Both A and R are true but R is not the explanation of A. (iii) A is true and R is false. (iv) Both A and R are false. ...
The effect of the uncertainty principle is significant only for the motion of microscopic particles and is negligible for the macroscopic particles. Justify the statement with the help of a suitable example.
The uncertainty principle is applicable only for microscopic particles and can be concluded from the uncertainty measurement. Example: Take a particle of mass = 1 milligram ∆x. ∆ν = 60626*10-34/...
Table-tennis ball has a mass 10 g and a speed of 90 m/s. If speed can be measured within an accuracy of 4% what will be the uncertainty in speed and position?
According to Heisenberg’s uncertainty principle: ∆x. ∆p ≥ h/4π Mass of the ball = 4 g Speed is = 90 m /s Uncertainity of speed, ∆v = 4/100 × 90 ∆v = 3.6 m/s ∆x = h/4πm∆v ∆x = 6.26 × 10-34 / 4 × 3.14...
What is the difference between the terms orbit and orbital?
Orbit represents a clear circular path for electrons to surround the nucleus. Represents the two-dimensional movement of electrons around the nucleus, the orbital is not that well defined because it...
Chlorophyll present in green leaves of plants absorbs light at 4.620 × 1014 Hz. Calculate the wavelength of radiation in nanometer. Which part of the electromagnetic spectrum does it belong to?
The relationship between the wavelength and the frequency: λ = c/ν c - Velocity of light v - Frequency of the radiation. λ = 3 x 108 ms-1 / 4.620 x 1014 Hz Hence, λ = 0.6494...
Out of electron and proton which one will have, a higher velocity to produce matter waves of the same wavelength? Explain it.
The electron which is a lighter particle will have the higher velocity and will also produce matter waves having the same wavelength. This is because, if the mass is less, then the velocity increases.
The Balmer series in the hydrogen spectrum corresponds to the transition from n1 = 2 to n2 = 3,4,………. This series lies in the visible region. Calculate the wavenumber of the line associated with the transition in Balmer series when the electron moves to n = 4 orbit. (RH= 109677 cm-1)
Calculation: According to Bohr’s model for the hydrogen atom; ν = RH(1/n12-1/ n22)cm-1 Given, n1 = 2 n2 = 4 H (Rydberg’s constant) = 109677 Wave number = 109677 ( ¼-1/16) Hence, Wave number =...
The electronic configuration of the valence shell of Cu is 3d10 4s1 and not 3d94s2. How is this configuration explained?
Great stability is established to the orbitals which are half or completely filled. In the given electronic configuration 3d104s1 of Copper (Cu), the stability is assured (d orbitals - filled, s...
Wavelengths of different radiations are given below :
λ(A) = 300 nm λ(B) = 300 μm λ(c) = 3 nm λ (D) 30 A° Arrange these radiations in the increasing order of their energies. Given, λ(A) = 300 nm λ(A) = 300 x 10-9 m λ(A) = 3 x 10 -7 m λ(B)...
An atom having atomic mass number 13 has 7 neutrons. What is the atomic number of the atom?
Calculation: Atomic mass number = number of protons + number of neutrons Number of protons = atomic mass number – number of neutrons. Hence, atomic number of an atom = 13 – 7 = 6.
Which of the following will not show deflection from the path on passing through an electric field? Proton, cathode rays, electron, neutron
Neutron shows no deflection from the path passing through the electric field. This is due to the neutrality of neutron particles. Therefore, it has no charge and is not affected by any electrical...
Calculate the total number of angular nodes and radial nodes present in 3p orbital.
The region where the probability of finding the electrons is zero, it is considered as the nodes and is it present among the orbitals. Example: In the np orbitals, Nodes = n – l – 1 Nodes = 3 –1 – 1...
Which of the following orbitals are degenerate? 3dxy, 4dxy, 3dz2 , 3dyx, 4dyx, 4dzz
The electron energy in a multielectron atom, in contrast to the hydrogen atom, depends not only on its quantum number, but also on its azimuthal quantum number. The same electron shells and the same...
Nickel atom can lose two electrons to form Ni2+ ion. The atomic number of nickel is 28. From which orbital will nickel lose two electrons.
1 Ni atom = 28 electrons and its electronic configuration is 4s2 3d8 It turns to Ni2+ by losing 2 electrons and its electronic configuration becomes 4s0 3d8 According to the Aufbau principle, Ni...
Show the distribution of electrons in oxygen atom (atomic number 8) using orbital diagram.
Distribution of electrons in oxygen atom: 1s22s22p4
Arrange s, p and d sub-shells of a shell in the increasing order of effective nuclear charge (Zeff) experienced by the electron present in them.
Arrangement of the subshells: d<p<s The s-orbitals shield the electrons a lot more when compared to the p-orbitals from the nucleus.
Which of the following statements concerning the quantum numbers are correct?
(i) The angular quantum number determines the three-dimensional shape of the orbital. (ii) The principal quantum number determines the orientation and energy of the orbital. (iii) The magnetic...
In which of the following pairs, the ions are iso-electronic?
(i) Na+, Mg2+ (ii) Al3+, O– (iii) Na+, O2- (iv) N3-, Cl– Correct Answers: (i) Na+, Mg2+ (iii) Na+, O2- Explanation: Isoelectronic species are the atoms / ions that has the same number...
Which of the following sets of quantum numbers is correct? n l m n l m
(i) 1 1 +2 (ii) 2 1 +1 (iii) 3 2 –2 (iv) 3 4 –2 Correct Answers: (ii) 2 1 +1 (iii) 3 2 –2 Explanation: The correct sets of quantum numbers are, n = 2, l = 1, m = +1 n = 3, l = 2, m =...
Out of the following pairs of electrons, identify the pairs of electrons present in degenerate orbitals :
(i) (a) n = 3, l = 2, ml = –2, ms= − ½ (b) n = 3, l = 2, ml = –1, ms= − 1/2 (ii) (a) n = 3, l = 1, ml = 1, ms = + ½ (b) n = 3, l = 2, ml = 1, ms = +1/2 (iii) (a) n = 4, l = 1, ml = 1, ms = +...
Identify the pairs which are not of isotopes?
(i) 6X12, 6Y13 (ii) 17X35, 6Y37 (iii) 6X14, 7Y14 (iv) 4X8, 5Y8 Correct Answers: (iii) 6X14, 7Y14 (iv) 4X8, 5Y8 Explanation: Isotopes are the atoms having same atomic number but...
If travelling at the same speeds, which of the following matter waves have the shortest wavelength?
(i) Electron (ii) An alpha particle (He2+) (iii) Neutron (iv) Proton Correct Answer: (ii) An alpha particle (He2+) Explanation: According to de Broglie's equation, the alpha particles...
For the electrons of an oxygen atom, which of the following statements is correct?
(i) Zeff for an electron in a 2s orbital is the same as Zeff for an electron in a 2p orbital. (ii) An electron in the 2s orbital has the same energy as an electron in the 2p orbital. (iii) Zeff for...
The pair of ions having same electronic configuration is __________.
(i) Cr3+, Fe3+ (ii) Fe3+, Mn2+ (iii) Fe3+, Co3+ (iv) Sc3+, Cr3+ Correct Answer: (ii) Fe3+, Mn2+ Explanation: Fe - Z=26 : 3d64s2 Fe3+- 3d5 Mn - Z=25 : 3d54s2 Mn2+ : 3d5 Hence,...
Number of angular nodes for 4d orbital is __________.
(i) 4 (ii) 3 (iii) 2 (iv) 1 Correct Answer: (iii) 2 Explanation: The Number of angular nodes = l (azimuthal quantum number) Hence, the number of angular nodes for 4d orbital is...
