If the threshold wavelength is $\lambda_{0} n m\left(=\lambda_{0} \times 10^{-9} \mathrm{~m}\right)$, the K.E. of the radiation would be: $ h\left(\nu-\nu_{0}\right)=\frac{1}{2} m \nu^{2} $ Three...
The work function for the caesium atom is 1.9 eV. Calculate
(a) the threshold wavelength and
(b) the threshold frequency of the radiation. If the caesium element is irradiated with a wavelength of 500 nm,(c) calculate the kinetic energy and the velocity of the ejected photoelectron.
Given, the work function of caesium $\left(W_{0}\right)=1.9 \mathrm{eV}$ (a)From the $W_{0}=\frac{h c}{\lambda_{0}}$ expression, we get: $\lambda_{0}=\frac{h c}{W_{0}}$ Where, $\lambda_{0}$ is the...
In astronomical observations, signals observed from the distant stars are generally weak. If the photon detector receives a total of  from the radiations of
from the radiations of 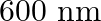 , calculate the number of photons received by the detector.
, calculate the number of photons received by the detector.
From the expression of energy of one photon (E), $ E=\frac{h c}{\lambda} $ Where, $\lambda$ denotes the wavelength of the radiation $\mathrm{h}$ is Planck's constant c denotes the velocity of the...
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate the number of quanta presents if it produces 2 J of energy.
No. quanta in $2 \mathrm{~J}$ of energy $\frac{2 J}{32.27 \times 10^{-20} J}$ $ =6.19 \times 10^{18} $ $ =6.2 \times 10^{18} $
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate the energy of quantum
Energy of one quantum $(E)=h v$ $ =\left(6.626 \times 10^{-34} \mathrm{Js}\right)\left(4.87 \times 10^{14} \mathrm{~s}^{-1}\right) $ Energy of one quantum $(\mathrm{E})=32.27 \times 10^{-20}...
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate distance travelled by this radiation in 30 s
Speed of the radiation, $\mathrm{c}=3 \times 10^{8} \mathrm{~ms}^{-1}$ Distance travelled by the radiation in a timespan of $30 \mathrm{~s}$ $ \begin{array}{l} =\left(3 \times 10^{8}...
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate the frequency of emission,
Wavelength of the emitted radiation $=616 \mathrm{~nm}=616 \times 10^{-9} \mathrm{~m}$ (Given) (a)Frequency of the emission $(\nu)$ $ \nu=\frac{c}{\lambda} $ Where, $c=$ speed of the radiation...
Nitrogen laser produces radiation at a wavelength of 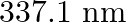 . If the number of photons emitted is
. If the number of photons emitted is 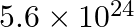 , calculate the power of this laser.
, calculate the power of this laser.
Energy with which it emits photons=Power of laser Power $=E=\frac{N h c}{\lambda}$ Where, $N=$ number of photons emitted $\mathrm{h}=$ Planck's constant $\mathrm{c}=$ velocity of radiation...
An ion with mass number 56 contains 3 units of positive charge and 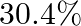 more neutrons than electrons. Assign the symbol to this ion.
more neutrons than electrons. Assign the symbol to this ion.
Let us consider the total no. electrons present in $A^{3+}$ be $x$. Now, total no. neutrons in it $=x+30.4 \%$ of $x=1.304 x$ Since the ion has a charge of $+3, \Rightarrow$ no. electrons in neutral...
An ion with mass number 37 possesses one unit of negative charge. If the ion contains 11.1% more neutrons than the electrons, find the symbol of the ion.
The number of electrons in a negatively charged ion be $x$. Then, no. neutrons present $=x+11.1 \%$ of $x=x+0.111 x=1.111 x$ No. electrons present in the neutral atom $=(x-1)$ (When an ion carries...
An element with mass number 81 contains 31.7% more neutrons as compared to protons. Assign the atomic symbol.
Let us consider that the No.of protons in the element be $x$. No. of neutrons $=x+31.7 \%$ of $x$ $=x+0.317 x$ $=1.317 x$ According to the question, Mass number of the element $=81$, which...
Symbols 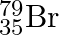 and
and 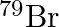 can be written, whereas symbols
can be written, whereas symbols 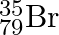 and
and 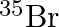 are not acceptable. Answer briefly.
are not acceptable. Answer briefly.
The general convention followed while representing elements along with their atomic masses (A), and their atomic numbers $(Z)$ is ${ }_{Z}^{A} \mathrm{X}$. Therefore, ${ }_{35}^{79} \mathrm{Br}$ is...
In Rutherford’s experiment, generally the thin foil of heavy atoms, like gold, platinum etc. have been used to be bombarded by the α-particles. If the thin foil of light atoms like Aluminium etc. is used, what difference would be observed from the above results?
The findings obtained with a foil made up of heavy atoms will differ from those obtained with a foil made up of comparatively light atoms. The magnitude of positive charge in the nucleus of a...
In Milikan’s experiment, the static electric charge on the oil drops has been obtained by shining 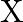 rays. If the static electric charge on the oil drop is
rays. If the static electric charge on the oil drop is  , calculate the number of electrons present on it.
, calculate the number of electrons present on it.
Charge held by the oil drop $=1.282 \times 10^{-18} C$ Charge held by one electron $=1.6022 \times 10^{-19} C$ Therefore, No. electrons present in the drop of oil $\frac{1.282 \times 10^{-18}...
A certain particle carries 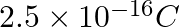 of static electric charge. Calculate the number of electrons present in it.
of static electric charge. Calculate the number of electrons present in it.
Charge held by one electron $=1.6022 \times 10^{-19} C \Rightarrow 1.6022 \times 10^{-19} C$ charge is held by one electron. Therefore, electrons carrying charge of $2.5 \times 10^{-16} C \quad...
The diameter of the zinc atom is 2.6Å. Calculate (a) radius of zinc atom in pm and (b) number of atoms present in a length of 1.6 cm if the zinc atoms are arranged side by side lengthwise.
Radius of carbon atom$ =\frac{2.6}{2} $ $ \begin{array}{l} =1.3 \times 10^{-10} \mathrm{~m} \\ =130 \times 10^{-12} \mathrm{~m}=130 \mathrm{pm} \end{array} $ (b) Length of the arrangement $=1.6...
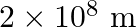 atoms of carbon are arranged side by side. Calculate the radius of carbon atom if th length of this arrangement is
atoms of carbon are arranged side by side. Calculate the radius of carbon atom if th length of this arrangement is 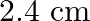 .
.
Length of the arrangement $=2.4 \mathrm{~cm}$ No. carbon atoms present $=2 \times 10^{8}$ The diameter of the carbon atom $=\frac{2.4 \times 10^{-2} m}{2 \times 10^{8} m}$ $=1.2 \times 10^{-10} m...
If the diameter of a carbon atom is 0.15 nm, calculate the number of carbon atoms which can be placed side by side in a straight line across the length of the scale of length 20 cm long.
We know that$ 1 \mathrm{~cm}=10^{-2} \mathrm{~m} $ Length of the scale $=20 \mathrm{~cm}=20 \times 10^{-2} \mathrm{~m}$ Diameter of one carbon atom $=0.15 \mathrm{~nm}=0.15 \times 10^{-9}...
Calculate the energy required for the process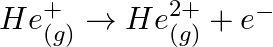 The ionization energy for the
The ionization energy for the  atom in the ground state is
atom in the ground state is 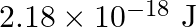 atom
atom 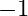
The energy associated with hydrogen-like species is: $ E_{n}=-2.18 \times 10^{-18}\left(\frac{Z^{2}}{n^{2}}\right) J $ For the ground state of the hydrogen atom, $ \begin{array}{l} \Delta...
What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition n = 4 to n = 2 of He+ spectrum?
The wave number associated with the Balmer transition for the He+ ion (n = 4 to n = 2 ) is given by: $ \bar{\nu}=\frac{1}{\lambda}=R Z^{2}\left(\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}}\right) $...
Show that the circumference of the Bohr orbit for the hydrogen atom is an integral multiple of the de Broglie wavelength associated with the electron revolving around the orbit.
Only one electron exists in hydrogen atoms. The angular momentum of this electron, according to Bohr's postulates, is: $m v r=n \frac{h}{2 \pi} \quad \ldots .(1)$ Where, $n=1,2,3, \ldots$ As per de...
How many electrons in an atom may have the following quantum numbers?a) n = 4, 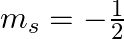 b)n = 3, l = 0
b)n = 3, l = 0
(a)The total number of electrons in the atom =$2n^2$ if n is the primary quantum number. Hence, For n = 4, Total no. electrons = 2 (16) = 32 An atom with 32 electrons has the following electron...
Using s, p and d notations, describe the orbital with the following quantum numbers.
(a)n = 1, l = 0;
(b)n = 3; l =1
(c) n = 4; l = 2;
(d) n = 4; l =3.
(a)n = 1, l = 0 implies a 1s orbital. (b)n = 3 and l = 1 implies a 3p orbital. (c)n = 4 and l = 2 implies a 4d orbital. (d)n = 4 and l = 3 implies a 4f orbital.
(I)An atomic orbital has n = 3. What are the possible values of l and ml ? (II)List the quantum numbers (ml and l) of electrons for 3d orbital. (III) Which of the following orbitals are possible? 1p, 2s, 2p and 3f
(I) The range of potential values for 'l' is 0 to (n – 1). As a result, the possible values of l for n = 3 are 0, 1, and 2. The total number of potential ml = (2l + 1) values. It has a range of...
Give the number of electrons in the species , 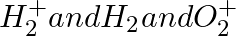
The number of electrons in H2 is 1 + 1 = 2. 2 – 1 = 1 number of electrons in H2+ H2: The number of electrons in H2 equals 1 + 1 = 2. Number of electrons in O2 = 8 + 8 = 16. In O2+, the number of...
An atom of an element contains 29 electrons and 35 neutrons. Deduce (i) the number of protons and (ii) the electronic configuration of the element.
(i)No.protons = no.electrons in a neutral atom. The number of protons in the atoms of the element is 29. (ii)The electronic configuration of this element (atomic number 29) is 1s...
An electron is in one of the 3d orbitals. Give the possible values of n, l and ml for this electron.
For the 3d orbital: Principal quantum number ,possible values (n) = 3 Azimuthal quantum number, possible values(l) = 2 Magnetic quantum number ,possible values(ml) = – 2, – 1, 0, 1, 2
(III) Which atoms are indicated by the following configurations? (a) ![Rendered by QuickLaTeX.com [\mathrm{He}]{2} \mathrm{~s}^{1}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-949e215e254ffdae25444046a6badfbf_l3.png)
 Ne]
Ne] 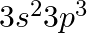 (c)
(c) ![Rendered by QuickLaTeX.com [\mathrm{Ar}] 4 \mathrm{~s}^{2} 3 \mathrm{~d}^{1}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c46b18eb49590f81f622e0747e88d18b_l3.png)
$(I I I)(a)[H e] 2 s^{1}$ Complete electronic configuration: $1 \mathrm{~s}^{2} 2 \mathrm{~s}{ }^{1}$. $\therefore$ the element's atomic number is 3 . The element is lithium (Li) (b) $[\mathrm{Ne}]...
(I)Write the electronic configurations of the following ions:(a) 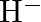 (b)
(b) 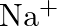 (c)
(c) 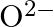 (d)
(d) 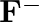
The electronic configuration of the Hydrogen atom (in its ground state) $1 \mathrm{~s}^{1}$. The single negative charge on this atom indicates that it has gained an electron. Hence, the electronic...
The mass of an electron is 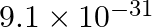 kg. If its K.E. is
kg. If its K.E. is J, calculate its wavelength.
J, calculate its wavelength.
As per de Broglie’s equation, $ \lambda=\frac{h}{m v} $ Given, K.E of electron $=3.0 \times 10^{-25} \mathrm{~J}$ $ \begin{array}{l} \text { Since K.E.= } \frac{1}{2} m v^{2} \therefore...
Calculate the wavelength of an electron moving with a velocity of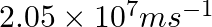
As per de Broglie’s equation, $ \lambda=\frac{h}{m v} $ Where, $\lambda$ denotes thr wavelength of the moving particle $\mathrm{m}$ is the mass of the particle $v$ denotes the velocity of the...
(i) The energy associated with the first orbit in the hydrogen atom is 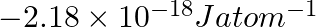 . What is the energy associated with the fifth orbit? (ii) Calculate the radius of Bohr’s fifth orbit for the hydrogen atom.
. What is the energy associated with the fifth orbit? (ii) Calculate the radius of Bohr’s fifth orbit for the hydrogen atom.
(i) Energy associated with the fifth orbit of hydrogen atom is calculated as: $E_{5}=\frac{-\left(2.18 \times 10^{-18}\right)}{(5)^{2}}=\frac{-\left(2.18 \times 10^{-18}\right)}{25}=-8.72 \times...
Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nanosecond range. If the radiation source has the duration of 2 ns and the number of photons emitted during the pulse source is 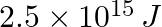 , calculate the energy of the source.
, calculate the energy of the source.
Frequency of radiation $\nu$ $\nu =\frac{1}{2.0\times 10^{-9}s}$ $\nu =5.0\times 10^{8}s^{-1}$ Energy (E) of source = Nhν Where, N is the no. photons emitted h is Planck’s constant ν denotes the...
The ejection of the photoelectron from the silver metal in the photoelectric effect experiment can be stopped by applying the voltage of 0.35 V when the radiation 256.7 nm is used. Calculate the work function for silver metal.
The energy associated with an incident photon (E) must equal the sum of its kinetic energy and the work function (W0) of the radiation, according to the rule of conservation of energy. E = W0 + K.E...
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 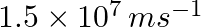 , calculate the energy with which it is bound to the nucleus.
, calculate the energy with which it is bound to the nucleus.
Energy of incident photon (E) is given by, $E=\frac{hc}{\lambda }$ $E=\frac{(6.626\times 10^{-34})(3\times 10^{8})}{(150\times 10^{-12})}=1.3252\times 10^{-15}\, J$ $\simeq 13.252\times 10^{-16}J$...
Emission transitions in the Paschen series end at orbit n = 3 and start from orbit n and can be represented as v =  (Hz) [1/3^2 – 1/n^ 2 ] Calculate the value of n if the transition is observed at 1285 nm. Find the region of the spectrum.
(Hz) [1/3^2 – 1/n^ 2 ] Calculate the value of n if the transition is observed at 1285 nm. Find the region of the spectrum.
Wavelength of the transition = 1285 nm =$1285 \times 10^{-9} m$(Given) $\nu =3.29\times 10^{15}(\frac{1}{3^{2}}-\frac{1}{n^{2}})$ Since$ \nu =\frac{c}{\lambda }$ =$\frac{3\times...
Calculate the wavelength for the emission transition if it starts from the orbit having radius 1.3225 nm and ends at 211.6 pm. Name the series to which this transition belongs and the region of the spectrum.
The radius of the n th orbit of hydrogen-like particles is given by, $r=\frac{0.529n^{2}}{Z}$ $r=\frac{5.29n^{2}}{Z}pm$ For radius (r1) = 1.3225 nm $=1.32225\times 10^{-9}m$ $=1322.25\times...
Dual behaviour of matter proposed by de Broglie led to the discovery of electron microscope often used for the highly magnified images of biological molecules and another type of material. If the velocity of the electron in this microscope is 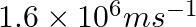 , calculate de Broglie wavelength associated with this electron.
, calculate de Broglie wavelength associated with this electron.
As per de Broglie’s equation, $\lambda =\frac{h}{m\nu }$ =$\frac{(6.626\times 10^{-34})}{9.103939\times 10^{-31}kg(1.6\times 10^{6}ms^{-1})}$ =$4.55\times 10^{-10}m\lambda =455pm$ Therefore, de...
Similar to electron diffraction, neutron diffraction microscope is also used for the determination of the structure of molecules. If the wavelength used here is 800 pm, calculate the characteristic velocity associated with the neutron.
From de Broglie’s equation, $\lambda =\frac{h}{m\nu }$ $\nu=\frac{h}{m\lambda}$ Where, v denotes the velocity of the neutron h is Planck’s constant m is the mass of the neutron λ is the wavelength...
If the velocity of the electron in Bohr’s first orbit is 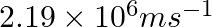 , calculate the de Broglie wavelength associated with it.
, calculate the de Broglie wavelength associated with it.
As per de Broglie’s equation, $\lambda =\frac{h}{m\nu}$ Where, λ is the wavelength of the electron h is Planck’s constant m is the mass of the electron v denotes the velocity of electron...
The velocity associated with a proton moving in a potential difference of 1000 V is 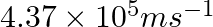 . If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
. If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
As per de Broglie’s expression, $\lambda =\frac{h}{m\nu }$ $ =\frac{(6.626\times 10^{-34})}{0.1kg(4.37\times 10^{5}ms^{-1})}$=$1.516\times 10^{-38}m$
If the position of the electron is measured within an accuracy of ± 0.002 nm, calculate the uncertainty in the momentum of the electron. Suppose the momentum of the electron is h/4πm × 0.05 nm, is there any problem in defining this value.
As per Heisenberg’s uncertainty principle, ∆x.∆p >= h/4π Where, ∆x = uncertainty in the position of the electron ∆p = uncertainty in the momentum of the electron Substituting the given values in...
The bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p orbital and 5 electrons in 4p orbital. Which of these electron experiences the lowest effective nuclear charge?
The nuclear charge that electrons (which are present in atoms containing multiple electrons) feel is determined by the distance between their orbital and the atom's nucleus. The smaller the...
Among the following pairs of orbitals which orbital will experience the larger effective nuclear charge? (i) 2s and 3s, (ii) 4d and 4f, (iii) 3d and 3p
The net positive charge acting on an electron in an atom's orbital with more than one electron is known as the nuclear charge. The distance between the orbital and the nucleus is inversely...
Indicate the number of unpaired electrons in: (a)P (b)Si (c)Cr (d)Fe (e)Kr
(a)Phosphorus (P): The atomic number of phosphorus is 15 Electronic configuration of Phosphorus: 1s 2 2s 2 2p 6 3s 2 3p 3 This can be represented as follows: From the diagram, it can be observed...
(a) How many sub-shells are associated with n = 4? (b) How many electrons will be present in the sub-shells having ms value of –1/2 for n = 4?
(a)n = 4 (Given) For some value of ‘n’, the values of ‘l’ range from 0 to (n – 1). Here, the possible values of l are 0, 1, 2, and 3 Therefore, a total of 4 subshells are possible when n=4: the s,...
What is the maximum number of emission lines when the excited electron of a H atom in n = 6 drops to the ground state?
A total number of 15 lines (5 + 4 + 3 + 2 + 1) will be obtained in this hydrogen emission spectrum. The total number of spectral lines emitted when an electron drops to the ground state from the...
How much energy is required to ionise a H atom if the electron occupies n = 5 orbit? Compare your answer with the ionization enthalpy of H atom (energy required to remove the electron from n =1 orbit).
The expression for the ionization energy is given by, $E_{n} =\frac{-(2.18\times 10^{-18})Z^{2}}{n^{2}}$ Where Z denotes the atomic number and n is the principal quantum number For the ionization...
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes the transition from an energy level with n = 4 to an energy level with n = 2?
The $n_{i} = 4$ to$ n_{f}$= 2 transition results in a spectral line of the Balmer series. The energy involved in this transition can be calculated using the following expression: $E=2.18\times...
Electrons are emitted with zero velocity from a metal surface when it is exposed to radiation of wavelength 6800 Å. Calculate threshold frequency (ν0 ) and work function (W0 ) of the metal.
Threshold wavelength of the radiation $(\lambda _{0})= 6800 Å=6800\times 10^{-10}\, m$ Threshold frequency of the metal $(\nu _{0}) =\frac{c}{\lambda _{0}}=$ $\frac{3\times 10^{8}ms^{-1}}{6.8\times...
A 25-watt bulb emits monochromatic yellow light of the wavelength of 0.57µm. Calculate the rate of emission of quanta per second.
Power of the bulb,$ P = 25 Watt = 25\, Js^{-1}$ Energy (E) of one photon= $h\nu =\frac{hc}{\nu} $ Substituting these values in the expression for E: $E=\frac{(6.626\times 10^{-34})(3\times...
Electromagnetic radiation of wavelength 242 nm is just sufficient to ionise the sodium atom. Calculate the ionisation energy of sodium in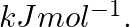
Ionization energy (E) of sodium =$ \frac{N_{A}hc}{\lambda }$ =$\frac{(6.023\times 10^{23}\, mol^{-1})(6.626\times 10^{-34})Js(3\times 10^{8})ms^{-1}}{242\times 10^{-9}m}$ =$4.947\times 10^{5}\, J\,...
A photon of wavelength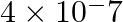 m strikes on metal surface, the work function of the metal is 2.13 eV. Calculate (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron
m strikes on metal surface, the work function of the metal is 2.13 eV. Calculate (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron 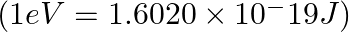 .
.
(i) Energy of the photon $(E)= h\nu =\frac{hc}{\lambda }$ Where, h denotes Planck’s constant, whose value is $6.626\times 10^{-34}\,Js$ c denotes the speed of light =$ 3\times 10^{8}\,m/s$...
What is the number of photons of light with a wavelength of 4000 pm that provides 1J of energy?
Energy of one photon (E) =$ h\nu$ Energy of ‘n’ photons $E_{n} = nh\nu \Rightarrow n=\frac{E_{n}\lambda }$ Where, \lambdaλ is the wavelength of the photons = 4000 pm = $4000\times 10^{-12}\, m$ c...
Find the energy of each of the photons which (i) correspond to light of frequency 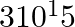 Hz. (ii) have a wavelength of 0.50 Å.
Hz. (ii) have a wavelength of 0.50 Å.
(i) The energy of a photon (E) can be calculated by using the following expression: $E= h\nu$ Where, ‘h’ denotes Planck’s constant, which is equal to $6.626\times 10^{-34}\, Js\nuν$ (frequency of...
Yellow light emitted from a sodium lamp has a wavelength (λ) of 580 nm. Calculate the frequency (ν) and wavenumber (ν ) of the yellow light.
Rearranging the expression, $\lambda =\frac{c}{\nu }$ the following expression can be obtained, $\nu =\frac{c}{ \lambda }$ ……….(1) Here, $\nu$ denotes the frequency of the yellow light c denotes...
Write the complete symbol for the atom with the given atomic number (Z) and atomic mass (A) (I)Z = 17, A = 35 (II)Z = 92, A = 233 (III)Z = 4, A = 9
(i)\({}_{17}^{35}C\) (ii)\({}_{92}^{233}C\) (iii)\({}_{4}^{9}C\)
(i) Calculate the total number of electrons present in one mole of methane. (ii) Find (a) the total number and (b) the total mass of neutrons in 7 mg of 14C. (Assume that mass of a neutron = 1.675 × 10–27 kg). (iii) Find (a) the total number and (b) the total mass of protons in 34 mg of NH3 at STP. Will the answer change if the temperature and pressure are changed?
(i) 1 molecule of methane contains 10 electrons (6 from carbon, 4 from hydrogen) Therefore, 1 mole of methane contains 10*NA = 6.022*1024 electrons. (ii) Number of neutrons in 14g (1 mol) of 14C =...
How many neutrons and protons are there in the following nuclei?
Ans: 24 is the mass number. The number of protons in an atom equals the number of atoms in the atom. Mass number – Atomic number = 24 – 12 = 12 neutrons Numerical value of mass nubmer = 56 Number...
How many neutrons and protons are there in the following nuclei?
Ans: Carbon-13 mass number = 13 Carbon atomic number = number of protons in a single carbon atom = 6 As a result, the total number of neutrons in a carbon atom is calculated as follows: mass number...
(i) Calculate the total number of electrons present in one mole of methane. (ii) Find (a) the total number and (b) the total mass of neutrons in 7 mg of 14C. (Assume that mass of a neutron = 1.675 × 10–27 kg). (iii) Find (a) the total number and (b) the total mass of protons in 34 mg of NH3 at STP. Will the answer change if the temperature and pressure are changed?
Ans: (i) 1 molecule of methane contains 10 electrons (6 from carbon, 4 from hydrogen) Therefore, 1 mole of methane contains 10*NA = 6.022*1024 electrons. (ii) Number of neutrons in 14g (1 mol)...
(i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons.
Ans: 1 electron weighs 9.109*10-31 kg. Therefore, number of electrons that weigh 1 g (10-3 kg) = 10-3kg/9.109*10-31 kg = 1.098*1027 electrons (ii) Mass of one mole of electrons = NA* mass of one...
Assertion (A): It is impossible to determine the exact position and exact the momentum of an electron simultaneously. Reason (R): The path of an electron in an atom is clearly defined.
(i) Both A and R are true and R is the correct explanation of A. (ii) Both A and R are true and R is not the correct explanation of A. (iii) A is true and R is false. (iv) Both A and R are false....
Assertion (A): All isotopes of a given element show the same type of chemical behaviour. Reason (R): The chemical properties of an atom are controlled by the number of electrons in the atom.
(i) Both A and R are true and R is the correct explanation of A. (ii) Both A and R are true but R is not the correct explanation of A. (iii) A is true but R is false. (iv) Both A and R are false....
Match species are given in Column 1 with the electronic configuration given in Column 2.
Column 1 Column 2 (i) Cr (a) [Ar]3d84s0 (ii) Fe2+ (b) [Ar]3d104s1 (iii) Ni2+ (c) [Ar]3d64s0 (iv) Cu (d) [Ar] 3d54s1 (e) [Ar]3d64s2 Column 1 Column 2 (i) Cr (d) [Ar]...
Match the following
Column 1 Column 2 (i) Photon (a)Value is 4 for N shell (ii) Electron (b)Probability density (iii) ψ2 (c)Always a positive value (iv) The principal quantum number n (d)Exhibits both momentum...
Match the following
Column 1 Column 2 (i) X-rays (a) ν = 100-0 104 Hz (ii) UV (b) ν = 1010Hz (iii) Long radio waves (c) ν = 1016 Hz (iv) Microwave (d) ν = 1018Hz Column 1 Column 2 (i) X-rays (d) ν = 1018Hz (ii) UV...
Match the following
Rule Principle (i) Hund’s Rule (a) No two electrons in an atom can have the same set of four quantum numbers. (ii) Aufbau Principle (b) Half-filled and filled orbitals have extra stability....
Match the quantum numbers with the information provided by these Quantum number Information provided
Column 1 Column 2 (i) Principal quantum number (a) orientation of the orbital (ii) Azimuthal quantum number (b) energy and size of orbital (iii) Magnetic quantum number (c) spin of an electron (iv)...
Match the following species with their corresponding ground state electronic configuration.
Atom / Ion Electronic configuration (i) Cu (a) 1s2 2s2 2p6 3s2 3p6 3d10 (ii) Cu2+ (b) 1s2 2s2 2p6 3s2 3p6 (iii) Zn2+ (c) 1s2 2s2 2p6 3s2 3p6 3d10 4s1 (iv) Cr3+ (d) 1s2 2s2 2p6 3s2 3p63d9 (e) 1s2...
The hydrogen atom has only one electron, so mutual repulsion between electrons is absent. However, in multielectron atoms mutual repulsion between the electrons is significant. How does this affect the energy of an electron in the orbitals of the same principal quantum number in multielectron atoms?
Hydrogen atom has only one electron, so the mutual repulsion between the electrons is non-existent. However, in multielectron atoms the interaction between electrons is important. This is because,...
A hypothetical electromagnetic wave is shown in Figure. Find out the wavelength of the radiation.
Distance between the two identical successive points in a wave is called the wavelength. Given, In the hypothetical wave, Wavelength, λ = 4 × 2.16 pm Hence, Wavelength, λ = 8.64 pm.
Chlorine exists in two isotopic forms, Cl-37 and Cl-35 but its atomic mass is 35.5. This indicates the ratio of Cl-37 and Cl-35 is approximately
(i) 1:2 (ii) 1:1 (iii) 1:3 (iv) 3:1 Correct Answer: (iii) 1:3 Explanation: Ratio of isotope abundance of Chlorine 37 to Chlorine 35 = A1:A2 Ratio of isotope abundance of Chlorine 37...
Orbital angular momentum depends on __________.
(i) l (ii) n and l (iii) n and m (iv) m and s Correct Answer: (i) l Explanation: Orbital angular momentum depends on the value of l which is referred to as the azimuthal quantum number.
A total number of orbitals associated with the third shell will be __________.
(i) 2 (ii) 4 (iii) 9 (iv) 3 Correct Answer: (iii) 9 Explanation: The total number of orbitals in nth shell = n2 Hence, the total number of orbitals associated with the third shell will...
The number of radial nodes for 3p orbital is __________.
(i) 3 (ii) 4 (iii) 2 (iv) 1 Correct Answer: (iv) 1 Explanation: Number of radial nodes for 3p orbital = 3−1−1 Number of radial nodes for 3p orbital = 3−2 Number of radial nodes for 3p...
Two atoms are said to be isobars if.
(i) they have the same atomic number but a different mass number. (ii) they have the same number of electrons but a different number of neutrons. (iii) they have the same number of neutrons but a...
Which of the following properties of an atom could be explained correctly by Thomson Model of an atom?
(i) Overall neutrality of atom. (ii) Spectra of a hydrogen atom. (iii) Position of electrons, protons and neutrons in an atom. (iv) Stability of atom. Correct Answer: (i) Overall...
Which of the following statements about the electron is incorrect?
(i) It is a negatively charged particle. (ii) The mass of an electron is equal to the mass of a neutron. (iii) It is a basic constituent of all atoms. (iv) It is a constituent of cathode rays....
Which of the following statement is not correct about the characteristics of cathode rays?
(i) They start from the cathode and move towards the anode. (ii) They travel in a straight line in the absence of an external electrical or magnetic field. (iii) Characteristics of cathode rays do...
The probability density plots of 1s and 2s orbitals are given in Figure:
The density of dots in a region represents the probability density of finding electrons in the region. Based on the above diagram which of the following statements is incorrect? (i) 1s and 2s...
Which of the following options does not represent ground state electronic configuration of an atom?
(i) 1s2 2s2 2p6 3s2 3p6 3d8 4s2 (ii) 1s2 2s2 2p6 3s2 3p6 3d9 4s2 (iii) 1s2 2s2 2p6 3s2 3p6 3d10 4s1 (iv) 1s2 2s2 2p6 3s2 3p6 3d5 4s1 Correct Answer: (ii) 1s2 2s2 2p6 3s2 3p6 3d9 4s2...
Which of the following conclusions could not be derived from Rutherford’s α -particle scattering experiment?
(i) Most of the space in the atom is empty. (ii) The radius of the atom is about 10–10 m while that of a nucleus is 10–15 m. (iii) Electrons move in a circular path of fixed energy called orbits....
