(i)
Energy of the photon ![]()
Where, h denotes Planck’s constant, whose value is ![]()
c denotes the speed of light =![]()
![]() = wavelength of the photon =
= wavelength of the photon =![]()
Substituting these values in the expression for E:
![]()
Therefore, energy of the photon =![]()
(ii)
The kinetic energy of the emission E_{k}Ek can be calculated as follows:
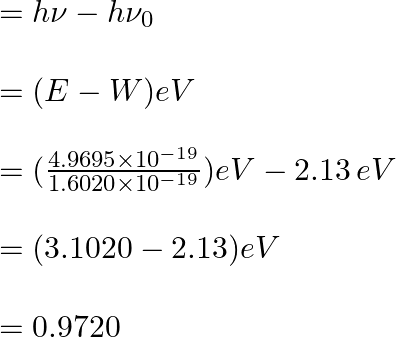
Therefore, the kinetic energy of the emission = 0.97 eV.
(iii)
The velocity of the photoelectron (v) can be determined using the following expression:
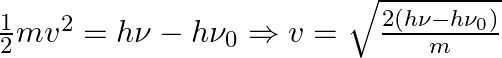
Where ![]() is the K.El of the emission (in Joules) and ‘m’ denotes the mass of the photoelectron.
is the K.El of the emission (in Joules) and ‘m’ denotes the mass of the photoelectron.
