
Ans:

Carbon-13 mass number = 13
Carbon atomic number = number of protons in a single carbon atom = 6
As a result, the total number of neutrons in a carbon atom is calculated as follows: mass number – atomic number = 13 – 6 = 7.
16 is the mass number of oxygen.
Number of protons in an atom of oxygen = 8
As a result, the number of neutrons equals the mass number minus the atomic number, which equals 16 – 8 = 8.


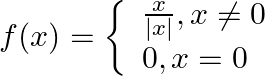 . Show that
. Show that 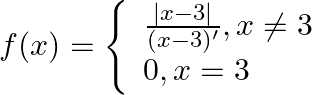 . Show that
. Show that