Answer:
The given reaction is:
![]()
The given concentration of various species is
![]() mol
mol![]()
At any given moment in time during a reaction, the reaction quotient (Q) quantifies the relative quantities of products and reactants present in the reaction mixture at that time.
Now, reaction quotient ![]() is:
is:
![Rendered by QuickLaTeX.com Q=\frac{\left[N H_{3}\right]^{2}}{\left[N_{2}]\left[H_{2}\right]^{3}\right.}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f66182803f75c4c44ec1abf450e3d2b2_l3.png)
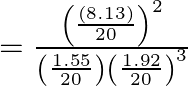
![]()
Since, ![]() , the reaction mixture is not at equilibrium.
, the reaction mixture is not at equilibrium.
As, ![]() . Hence, the reaction will move in the reverse direction.
. Hence, the reaction will move in the reverse direction.
