Solution:
For S and S2-
A sulphur atom has only 6 valence electrons, which is a very small number. As a result, the Lewis dot symbol for the letter S is

The presence of a bi-negative charge on sulphur indicates that it has gained two electrons since its formation. As a result, six valance electrons plus two electrons gained an electron each.
As a result, the Lewis dot symbol is

For Al and Al3+
The valence electrons in an aluminum atom are only three in number. As a result, the Lewis dot symbols for Al are as follows:

The presence of a tri-positive charge on aluminum indicates that it has donated three electrons to the process.
As a result, the Lewis dot symbol is

For H and H–
A hydrogen atom has only one valence electron, which is the most common type of electron. As a result, the Lewis dot symbol for the letter H is 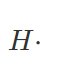
The fact that hydrogen has a single negative charge indicates that it has gained one electron. As a result, one valance electron was gained in addition to one gained electron.
As a result, the Lewis dot symbol is

