1:Answer is (A)
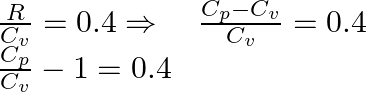
![]()
As ![]() the gas is diatomic in nature. For example air molecules.
the gas is diatomic in nature. For example air molecules.
For a gas 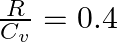 where ‘R’ is the universal gas constant and ‘
where ‘R’ is the universal gas constant and ‘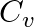 ’ is molar specific heat at constant volume. The gas is made up of molecules which are
’ is molar specific heat at constant volume. The gas is made up of molecules which are
A) rigid diatomic
B) monoatomic
C) non-rigid diatomic
D) polyatomic
A) rigid diatomic
B) monoatomic
C) non-rigid diatomic
D) polyatomic
For a gas 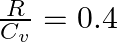 where ‘R’ is the universal gas constant and ‘
where ‘R’ is the universal gas constant and ‘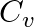 ’ is molar specific heat at constant volume. The gas is made up of molecules which are
’ is molar specific heat at constant volume. The gas is made up of molecules which are
A) rigid diatomic
B) monoatomic
C) non-rigid diatomic
D) polyatomic
A) rigid diatomic
B) monoatomic
C) non-rigid diatomic
D) polyatomic
