If ![]() is the radius of the atom then the volume of each atom will be
is the radius of the atom then the volume of each atom will be ![]()
Volume of all the substance will be ![]()
![]() atomic mass of the substance
atomic mass of the substance
![]() density of the substance
density of the substance
We know, One mole of the substance has ![]() atoms
atoms
![]()
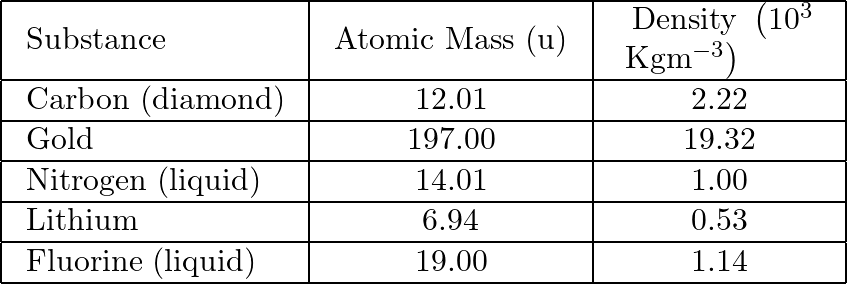
For carbon, ![]() and
and ![]()
![]()
![]()
![]()
For gold, ![]() and
and ![]()
![]()
![]()
For lithium, ![]() and
and ![]()
![]()
![]()
For nitrogen (liquid), ![]() and
and ![]()
![]()
![]()
For fluorine (liquid), ![]() and
and ![]()
![]()
![]()