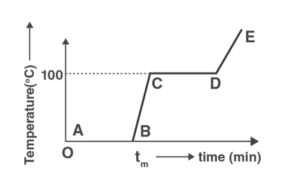
a) the region AB represents ice and water in thermal equilibrium
b) at B water starts boiling
c) at C all the water gets converted into steam
d) C to D represents water and steam in equilibrium at boiling point
Answer:
The correct options are
a) the region AB represents ice and water in thermal equilibrium
d) C to D represents water and steam in equilibrium at boiling point
Explanation:
Because the temperature is on the y axis and time is on the X-axis, there will be a change in temperature only if the graph has a slope, and there will be no change in temperature if the graph does not have a slope. There will be no change in the status of ice if there is no slope.
a step-by-step procedure
By looking at the graph AB, we can see that there is no change in temperature, which is visible in the graph, hence the state of ice will change, i.e. ice will melt (from solid-state to liquid state).
BC – We can clearly see the temperature shift, indicating that the water temperature is presently rising.
CD – We can clearly observe that the temperature has not changed, indicating that the water condition has changed this time (from liquid state to gaseous state).
Now, in DE, we can clearly see that the temperature has shifted. As a result, the temperature of the vapor has changed.
Considering option (a)->AB, ice, and water are no longer in thermal equilibrium.
So, according to AB, there is a condition of equilibrium in the state of ice and water, with a reduction in temperature resulting in the production of ice and an increase in temperature resulting in the development of water.
As a result, option(a) is incorrect, as it implies that AB indicates that it is not in equilibrium.
Consider option (b): At B, the water begins to boil.
As a result, this choice is likewise erroneous, as water begins to boil at 100 degrees Celsius, or point C.
Consider option (c): At C, all of the water is transformed to steam.
As a result, this alternative is also erroneous, because at point D, water will be turned to steam.
Consider option (d), where CD denotes water and steam in equilibrium at the boiling point.
As a result, an increase in temperature causes the development of vapour, whereas a fall in temperature causes the formation of water.
The correct answer is (d).
