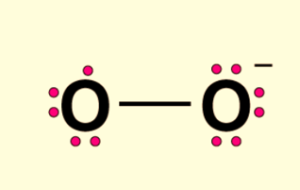Answer:
Because an oxygen atom with zero charges contains six electrons, the oxidation state of the atom is zero. An atom of oxygen with a negative charge contains seven electrons, while when it has a positive charge, it has six electrons. As a result, the oxidation state is one. The average oxidation state is equal to 0 + (-1)/2 = -1/2 on the oxidation scale.