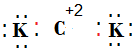A. Lithium B. Carbon
C. Fluorine D. Neon
(b) Group No. ’s
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Li | D | O | J | Ne | |||
| A | Mg | E | Si | H | K | ||
| B | C | F | G | L |
Select from the table:
(i) Which is the most electronegative?
(ii) How many valence electrons are present in G?
(iii) Write the formula of the compound between B and H.
(iv) In the compound between F and J, what type of bond will be formed?
(v) Draw the electron dot structure for the compound formed between C and K.
Answers:
(a) Correct Answer: A. Lithium
When we go from left to right in a period, the electron affinity decreases.
(b)
(i) The element J is the most electronegative.
(ii) 5 is the number of valence electron present in the element G.
(iii) The formula of the compound between B and H is B2H.
(iv) Covalent bond is formed between F and J.
(v)