(b) There are three elements E, F, G with atomic number 19, 8 and 17, respectively. Give the molecular formula of the compound formed between E and G and state the type of chemical bond in this compound.
Answers:
(a)
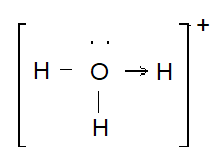
Type of bond present – Coordinate and covalent bond
(b) The valency Element E: +1
The valency Element G: -1
Molecular formula of its compound will be EG
Type of bond present – Ionic bond
