Wavelength of an electron is represented by ![]() and that of a photon by
and that of a photon by ![]() ,
,
So,
![]()
Planck’s constant, ![]()
For an electron having momentum ![]() , the kinetic energy (K) is given by the relation:
, the kinetic energy (K) is given by the relation:
![]()
Where, ![]() Mass of the electron
Mass of the electron ![]()
![]()
Therefore, 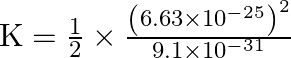
![]()
![]()
The kinetic energy of the electron is ![]() .
.
