Electrons are accelerated by a voltage of ![]()
Charge on an electron, ![]()
Mass of the electron, ![]()
Wavelength of the yellow light is given as ![]()
The kinetic energy of the electron, ![]()
![]()
![]()
De Broglie wavelength of electron is given as
![]()
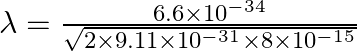
![]()
The wavelength of the blue light is ![]() times shorter than that of the yellow light. The wavelength of the light used and the resolving power of the microscope are inversely related. As a result, the electron microscope’s resolving power is
times shorter than that of the yellow light. The wavelength of the light used and the resolving power of the microscope are inversely related. As a result, the electron microscope’s resolving power is ![]() times larger than that of an optical microscope.
times larger than that of an optical microscope.
