(i) There is no solvation effect in the gas phase. As a result, the + I impact is primarily responsible for the fundamental strength. The firmer the base, the larger the +I impact. In addition, the + I affect increases as the amount of alkyl groups increases. As a result, in the gas phase, the following compounds can be grouped in decreasing order of their basic strengths:

(ii) The boiling point of a chemical is determined by the amount of H-bonding present. The boiling point of a compound increases as the H – bonding in the compound becomes more extensive. C2H5NH2 has two H atoms, whereas ( CH3)2NH has only one. As a result, C2H5NH2 experiences more H-bonding than (CH3)2NH. As a result, C2H5NH2 has a higher boiling point than (CH3)2NH.
In addition, O has a higher electronegative charge than N. C2H5OH, on the other hand, generates stronger H – bonds than C2H5NH2. As a result, C2H5OH has a higher boiling point than (C2H5)2NH & (CH3)2NH.
Based on the description above, the compounds in the question can be sorted in ascending order of their boiling points, as shown below:
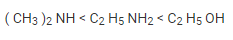
(iii) The higher the solubility, the more extensive the H – bonding. C6H5NH2 has two hydrogen atoms, whereas (C2H5)2NH has only one. As a result, C2H5NH has more H–bonding than (C2H5)2NH. As a result, C2H5NH has a higher solubility in water than (C2H5)2NH.
Furthermore, when the molecular mass of an amine increases, its solubility drops. This is due to the fact that the size of the hydrophobic portion of an amine increases its molecular mass. C6H5NH2 has a higher molecular mass than C2H5NH2 and (C2H5)2NH.
