Potential difference is given in the question as ![]()
Planck’s constant, ![]()
Mass of an electron, ![]()
Charge on an electron, ![]()
(a) The kinetic energy of each electron is equal to the accelerating potential t equilibrium,so we can write the relation of velocity (v) of each electron as:
![]()
![]()
Therefore, 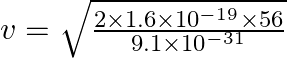
![]()
![]()
The momentum of each accelerated electron is given as:
![]()
![]()
Therefore, the momentum of each electron is ![]() .
.
(b) Through a potential ![]() , de Broglie wavelength of an electron accelerating is given by the relation:
, de Broglie wavelength of an electron accelerating is given by the relation:
![]()
![]()
As a result, ![]() is the de Broglie wavelength of each electron.
is the de Broglie wavelength of each electron.
