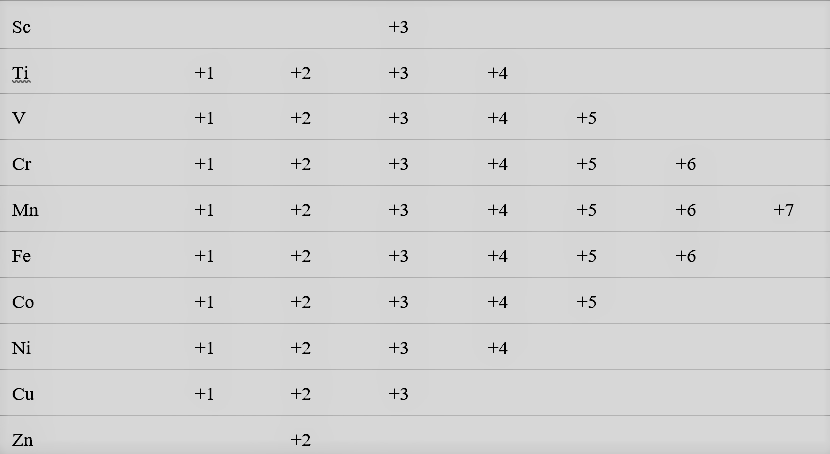
From the table shown above we can deduce the following points:
- Mn shows maximum number of oxidation states, that ranges between +2 to +7.
- The number of oxidation states increases as we move on from Sc to Mn, that is, from top to bottom till Mn shows up.
- On moving from Mn to Zn, the number of oxidation states decreases due to a decrease in the number of available unpaired electrons.
- The relative stability of the +2 oxidation state increases on moving from top to bottom. This is because on moving from top to bottom in a group, it becomes more and more difficult to remove the third electron from the d-orbital as it is tightly bound to the nucleus and requires a lot of energy.
