For Electrons, we have the relation for kinetic energy as,![]()
![]()
![]()
![]()
![]()
![]()
![]()
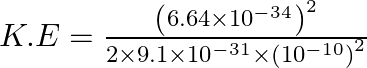
![]()
![]()
![]()
For photon of ![]() -rays, the expression for energy is,
-rays, the expression for energy is,![]()
![]()
![]()
![]()
The energy of X-ray photons is greater than that of the electron.
For Electrons, we have the relation for kinetic energy as,![]()
![]()
![]()
![]()
![]()
![]()
![]()
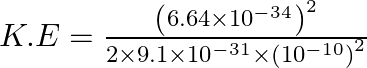
![]()
![]()
![]()
For photon of ![]() -rays, the expression for energy is,
-rays, the expression for energy is,![]()
![]()
![]()
![]()
The energy of X-ray photons is greater than that of the electron.