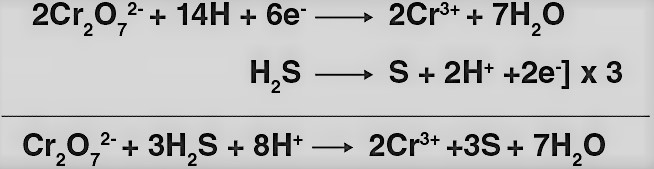
In acidic medium, K2Cr2O7 behaves as a very strong oxidising agent.
![]()
K2Cr2O7 gains electrons to get reduced and behaves as an oxidizing agent. The reaction of K2Cr2O7 with H2S, iron (II) solution and other iodide are as follows:
(i) K2Cr2O7 oxidizes iodide to iodine.
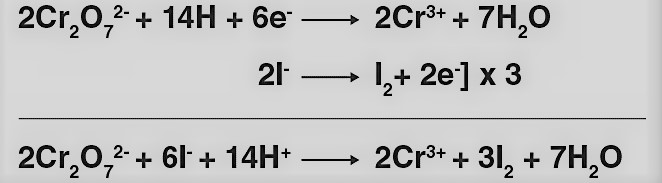
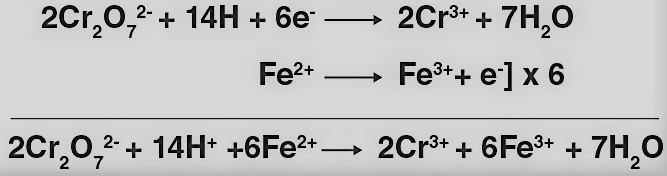
(ii) K2Cr2O7 oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
K2Cr2O7 oxidizes H2S to sulphur.