(i) Here, the oxidation state of cobalt is +3.
Orbitals of Co 3+ ion :
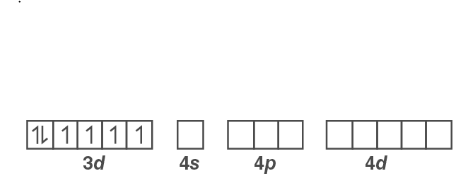
Oxalate is a field ligand with a low affinity. As a result, the electrons in the 3d orbital will not pair.
Because there are six ligands, hybridization must be either sp3d2or d2sp3.
Co3+ sp3d2 hybridization:
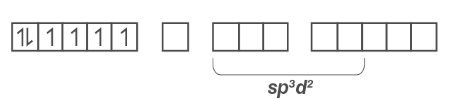
The 6 electron pairs from the 3 oxalate ions (oxalate anion is a bidentate ligand) occupy these sp3d 2 orbitals :
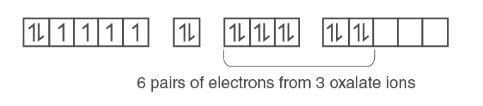
Therefore, the complex shows octahedral geometry.
(iv) In this complex, Cobalt has an oxidation state of +3.
Orbitals of Co3+ ion:
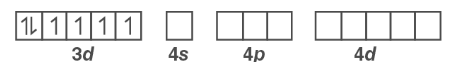
The 3d electrons will not pair because the fluoride ion is a weak field ligand. As a result, the Co3+ ion will undergo sp3d2 hybridization.
sp3d2 hybridized orbitals of Co3+ ion are :
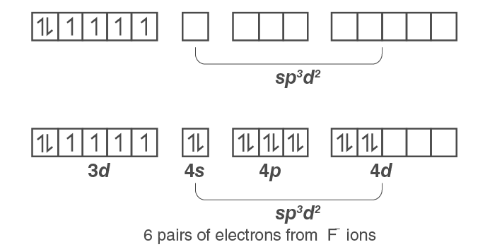
Therefore, the complex has a geometric configuration of an octahedral and it is paramagnetic.
