In the given table, we can find the oxidation numbers of the elements in the first half of the first row of transition elements with increasing atomic number:
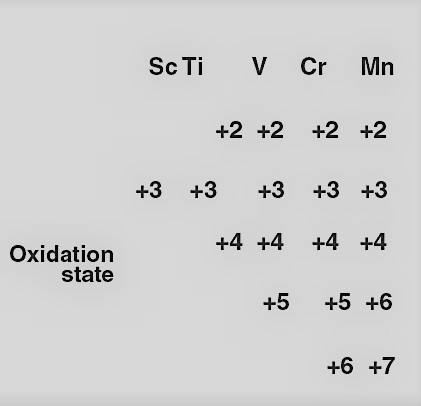
Here, we can find that except Sc all other metals have a +2 oxidation state. With an increase in atomic number from 21 to 25, i.e., from Sc to Mn, there is an increase in number of electrons from 1 to 5.
Sc (+2) = d1
Ti (+2) = d2
V (+2) = d3
Cr (+2) = d4
Mn (+2) = d5 When these metals lose two electrons from their 4s orbital, they attain +2 oxidation state. Since the number of d electrons in (+2) oxidation state also increases from Ti(+2) to Mn(+ 2), the stability of +2 state increases (as d-orbital is attaining more and more closer to half-filled stability). Mn (+2) has d5 electrons (that is half-filled d orbital, which is highly stable).
