Room temperature is given as ![]()
Atmospheric pressure is given as ![]()
Atomic weight of He atom as we know is ![]()
Avogadro’s number is ![]()
Boltzmann’s constant is ![]()
The expression for De Broglie wavelength is given as,
![]()
![]()
![]() mass of the He atom
mass of the He atom
![]() Atomic weight
Atomic weight ![]()
![]()
![]()
![]()
![]()
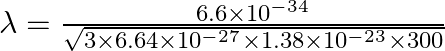
![]()
We have the ideal gas formula
![]()
![]()
![]()
Here,
![]() is the volume of the gas
is the volume of the gas
![]() is the number of moles of the gas
is the number of moles of the gas
Mean separation between the two atoms of the gas is given as
![]()
![Rendered by QuickLaTeX.com r=\left[\frac{1.38 \times 10^{-23} \times 300}{1.01 \times 10^{5}}\right]^{1 / 3}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-8c5e5a4097e038bd0701ba3f1996ebd5_l3.png)
![]()
The mean separation between the atom is greater than the de Broglie wavelength.
