(i) Ethylamine and aniline (ii) Aniline and benzylamine
(iii) Aniline and N-methylaniline.
(i) azo–dye test can distinguish aniline & Ethylamine.
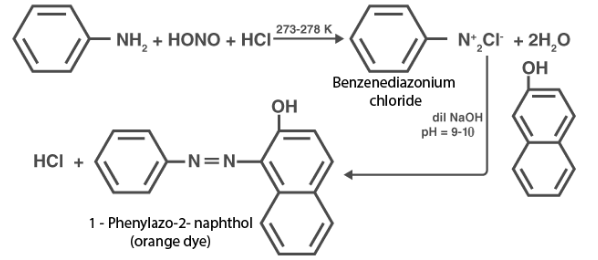
When aromatic amines react with HNO2 ( NaNO2 + dil. HCl ) at 0 – 5 ° C, a dye is formed which is then used to react with a 2 – naphthol alkaline solution. The dye is yellow, red, or orange in color. Aliphatic amines produce a brisk effervescence as a result of the production of Nitrogen gas under similar conditions.
(ii) Benzylamine and aniline can be distinguished by reacting with nitrous acid which is generated in situ from sodium nitrite and mineral acid. When nitrous acid reacts with benzylamine, an unstable diazonium salt is produced, along with an alcohol byproduct and the release of N2 gas.
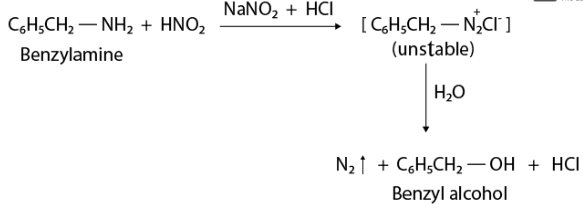
In another case, at a very low temperature, HNO2 reacts with aniline to produce a stable diazonium salt. As a result, there is no evolution of nitrogen gas.
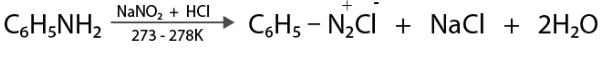
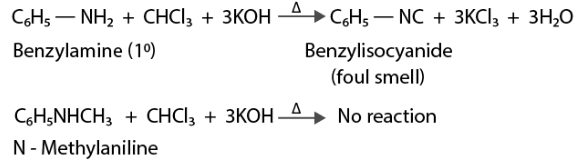
(iii) N – methylaniline & Aniline can be distinguishedhed by using the Carbylamine test.
On heating, the reaction of primary amines with ethanol, chloroform, and potassium hydroxide produces foul-smelling isocyanides or carbylamines. Aniline produces a positive carbylamines test because it is a primary aromatic primary. However, because N-methyl aniline is a secondary amine, it will not result in a positive carbylamines test.