Option A Cannot be predicted
Option B P2 =P1
Option C P2 > P1
Option D P2 < P1
Solution:
The correct option is Option D
According to the ideal gas equation,
PV =nRT
or we can write: V = nRT/P
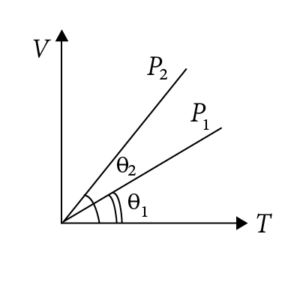
We know that for an isobaric process, P = constant and V ∝ T Therefore, V-T graph is a straight line passing through the origin and the slope of this line is inversely proportional to P.
In the given figure, we can see that
(Slop)2 > (Slop)1
Therefore, P2 < P1