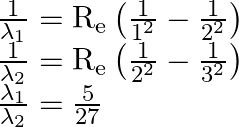In the spectrum of hydrogen, the ratio of the longest wavelength in the Lyman series to the longest wavelength in the
Balmer series is:
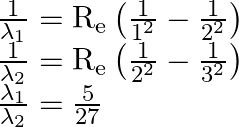
In the spectrum of hydrogen, the ratio of the longest wavelength in the Lyman series to the longest wavelength in the
Balmer series is: