The various types of isomerism that can be observed in coordination compounds are :
( i ) Geometrical isomerism :
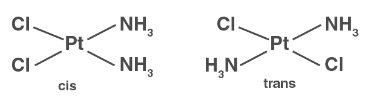
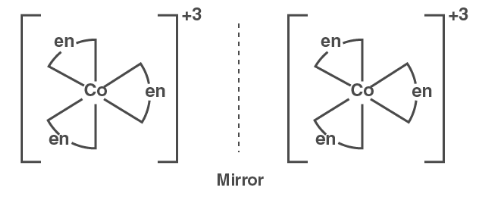
( ii ) Optical isomerism :
(iii) Coordination isomerism :
This kind of isomerism comes up when ligands are interchanged between anionic and cationic entities of different metal ions present in the complex.
Example – [ Cr( NH3)6] [ Co( CN )6]
(iv)
Linkage isomerism: This is found in complexes that have ambidentate ligands. For e.g. :[ Co( NH3 )5( NO2) ]Cl2 and [ Co( NH3)5( ONO) ]Cl2
(v) Ionisation isomerism: It is a type of isomerism in which a counter ion replaces a ligand within the coordination sphere.
e.g., [ Co( NH3)5Br ]SO4and [ Co( NH3)5SO4 ]Br
(vi) Solvate isomerism :
[ Cr( H2O)5 Cl ]Cl.H2O
