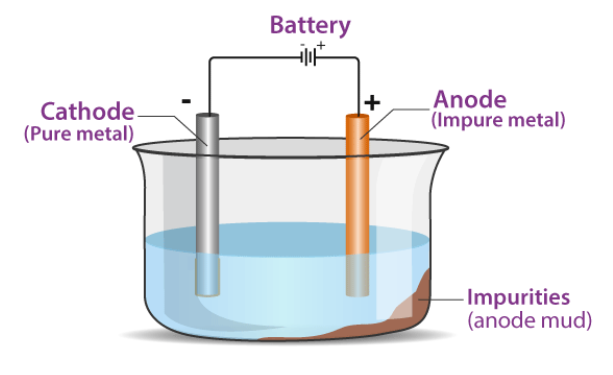
It is the process of employing electricity to refine impure metals. The anode is impure metal, and the cathode is a thin sheet of pure metal in this process. The electrolyte is a metal-specific salt solution.
When an electric current is passed through the electrolyte, metal ions from the electrolyte accumulate as pure metal at the cathode, while impure metal from the anode dissolves as ions in the solution ( electrolyte). The metal’s impurities settle below the anode. The anode mud is what it’s called.