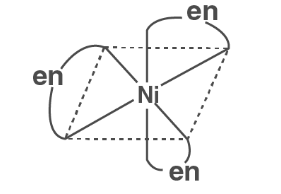
The metal-ligand bond becomes more stable when a polydentate or bidentate ligand attaches to a metal ion in such a way that it takes on the structure of a ring. Chelate rings are the name for these rings.
We can deduce from this that complexes containing chelate rings are more stable than complexes lacking the rings. The chelate effect is the name given to this phenomenon.
Ni2+ (aq) + 6NH3 ↔ [ Ni( NH3)6 ]2+ (aq)
logβ = 7.99
Ni2+ (aq) + 3en (aq) ↔ [ Ni( en)3 ]2+ (aq)
logβ = 18.1 ( more stable )