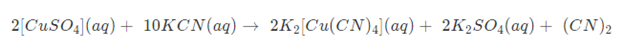
As a result of the foregoing procedure, the coordination entity obtained is K2[Cu(CN)4 ].
The above coordination entity does not ionize to produce Cu2+ ions since it is extremely stable. When hydrogen Sulphide gas is bubbled through it, no precipitate forms.
