Temperature of the nitrogen molecule is given as ![]()
Atomic mass of nitrogen is ![]()
Hence, mass of the nitrogen molecule will be,![]()
But, ![]()
Planck’s constant, ![]()
Boltzmann constant, ![]()
The expression that relates mean kinetic energy ![]() of the nitrogen molecule with the root mean is:
of the nitrogen molecule with the root mean is:
![]()
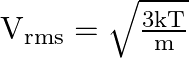
![]()
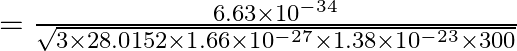
![]()
Therefore, ![]() is the de Broglie wavelength of the nitrogen molecule.
is the de Broglie wavelength of the nitrogen molecule.
