The Gabriel phthalimide synthesis is most commonly employed to make aliphatic primary amines. Nucleophilic substitution (SN2) of alkyl halides by the anion generated by the phthalimide is an example of this.
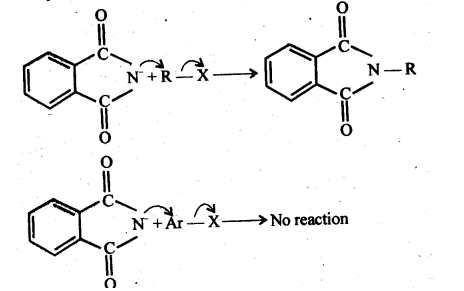
Aryl halides, on the other hand, do not undergo nucleophilic substitution with the anion produced by the phthalimide.

Therefore, Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
