solution:
Let the heaviness of dihydrogen be 20 g.
Let the heaviness of dioxygen be 80 g.
No. of moles of dihydrogen (nH2),
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} ~ \\ =\text{ }20/2 \\ ~ \\ =\text{ }10\text{ }moles \\ ~ \\ No.\text{ }of\text{ }moles\text{ }of\text{ }dioxygen\text{ }\left( nO2 \right), \\ ~ \\ =\text{ }80/32 \\ ~ \\ =\text{ }2.5\text{ }moles \\ ~ \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-3016dcefcc96ffdf3368c87879955ca8_l3.png)
Given:
![]()
Hence, halfway tension of dihydrogen (pH2),
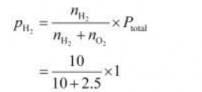
![]()
Hence, the halfway tension of dihydrogen is 0.8 bar.
solution:
Let the heaviness of dihydrogen be 20 g.
Let the heaviness of dioxygen be 80 g.
No. of moles of dihydrogen (nH2),
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} ~ \\ =\text{ }20/2 \\ ~ \\ =\text{ }10\text{ }moles \\ ~ \\ No.\text{ }of\text{ }moles\text{ }of\text{ }dioxygen\text{ }\left( nO2 \right), \\ ~ \\ =\text{ }80/32 \\ ~ \\ =\text{ }2.5\text{ }moles \\ ~ \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-3016dcefcc96ffdf3368c87879955ca8_l3.png)
Given:
![]()
Hence, halfway tension of dihydrogen (pH2),
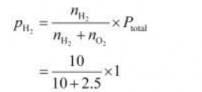
![]()
Hence, the halfway tension of dihydrogen is 0.8 bar.