(a) ![]()
Mass percent of 15 ppm chloroform in H2O= ![]()
![]()
![]() 100 grams of the sample is having 1.5 × g of
100 grams of the sample is having 1.5 × g of
![]()
∴ ![]()
= 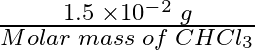
Molar mass (![]() )
)
= 12 + 1 + 3 (35.5)
= 119.5 grams ![]()
Therefore, molality of ![]() water
water
= ![]() m
m
(a) ![]()
Mass percent of 15 ppm chloroform in H2O= ![]()
![]()
![]() 100 grams of the sample is having 1.5 × g of
100 grams of the sample is having 1.5 × g of
![]()
∴ ![]()
= 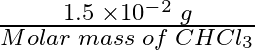
Molar mass (![]() )
)
= 12 + 1 + 3 (35.5)
= 119.5 grams ![]()
Therefore, molality of ![]() water
water
= ![]() m
m