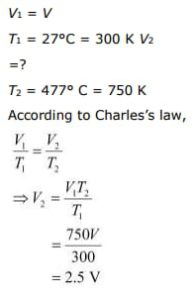solution:
Leave the volume of the round lined cup alone V.
Then, at that point, the volume of air inside the cup at 27° C is V.
Presently,
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} ~ \\ V1\text{ }=\text{ }V \\ ~ \\ T1\text{ }=\text{ }27{}^\circ C\text{ }=\text{ }300\text{ }K \\ ~ \\ V2\text{ }=? \\ ~ \\ T2\text{ }=\text{ }477{}^\circ \text{ }C\text{ }=\text{ }750\text{ }K \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c8b6b3ebe2e9b200900523d1f131c6c1_l3.png)
As per Charles’ law,
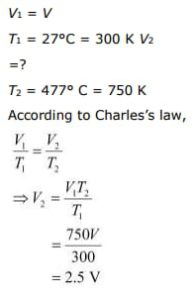

solution:
Leave the volume of the round lined cup alone V.
Then, at that point, the volume of air inside the cup at 27° C is V.
Presently,
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} ~ \\ V1\text{ }=\text{ }V \\ ~ \\ T1\text{ }=\text{ }27{}^\circ C\text{ }=\text{ }300\text{ }K \\ ~ \\ V2\text{ }=? \\ ~ \\ T2\text{ }=\text{ }477{}^\circ \text{ }C\text{ }=\text{ }750\text{ }K \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c8b6b3ebe2e9b200900523d1f131c6c1_l3.png)
As per Charles’ law,