Solution: Given, ![]()
![]()
As we know, molar conductivity,
Substituting the value of known parameters we have,
![Rendered by QuickLaTeX.com \[\begin{aligned}&=\frac{7.896 \times 10^{-5} S cm ^{-1}}{0.00241 mol L^{-1}} \times \frac{1000 cm ^{3}}{L} \&=32.76 S cm ^{2} mol ^{-1}\end{aligned}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-93dfc7f898ef77865b24196253fa1ef7_l3.png)
Also, given in question,
![]()
We know that,
![Rendered by QuickLaTeX.com \[\begin{aligned}&\alpha=\frac{\Lambda_{m}}{\Lambda_{m}^{0}} \&=\frac{32.76 S cm ^{2} mol ^{-1}}{390.5 Scm ^{2} mol ^{-1}}\end{aligned}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-d05561d4122763545cb882bb73a2c461_l3.png)
![]()
We know that, Dissociation constant,
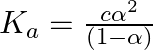
![Rendered by QuickLaTeX.com \[\begin{aligned}&=\frac{\left(0.00241 mol L^{-1}\right)(0.084)^{2}}{(1-0.084)} \&=1.86 \times 10^{-5} mol L ^{-1}\end{aligned}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f3cc521097764f9d2fe4a832f13c8cc0_l3.png)
Solution: Given, ![]()
![]()
Substituting the value of known parameters we have,
![Rendered by QuickLaTeX.com \[\begin{aligned}&=\frac{7.896 \times 10^{-5} S cm ^{-1}}{0.00241 mol L^{-1}} \times \frac{1000 cm ^{3}}{L} \&=32.76 S cm ^{2} mol ^{-1}\end{aligned}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-93dfc7f898ef77865b24196253fa1ef7_l3.png)
![]()
![Rendered by QuickLaTeX.com \[\begin{aligned}&\alpha=\frac{\Lambda_{m}}{\Lambda_{m}^{0}} \&=\frac{32.76 S cm ^{2} mol ^{-1}}{390.5 Scm ^{2} mol ^{-1}}\end{aligned}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-d05561d4122763545cb882bb73a2c461_l3.png)
![]()
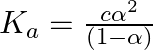
![Rendered by QuickLaTeX.com \[\begin{aligned}&=\frac{\left(0.00241 mol L^{-1}\right)(0.084)^{2}}{(1-0.084)} \&=1.86 \times 10^{-5} mol L ^{-1}\end{aligned}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f3cc521097764f9d2fe4a832f13c8cc0_l3.png)