In the cell reaction Cu + 2Ag+→ 2Ag + Cu2+, Anode is the oxidation half-cell Reaction - Cu → Cu2+ + 2e- Cathode is the reduction half-cell Reaction - 2Ag+ + 2e- →2Ag
Predict the products of electrolysis in each of the following:
(iii) A dilute solution of 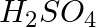 with platinum electrodes.
with platinum electrodes.
(iv) An aqueous solution of 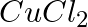 with platinum electrodes.
with platinum electrodes.
Solution: (iii) At the cathode, the following reduction reaction occurs to produce $H_{2}$ gas. $$ H{(a q)}^{+}+e^{-} \rightarrow \frac{1}{2} H_{2(g)}$$At the anode, the following processes are...
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of 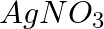 with silver electrodes. (ii) An aqueous solution of
with silver electrodes. (ii) An aqueous solution of 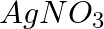 with platinum electrodes.
with platinum electrodes.
Solution: (i) At the cathode: The following reduction reactions are in competition with one another for space at the cathode.$$A g_{(a q)}^{+}+e^{-} \rightarrow A g_{(s)} ; E ^{0}=0.80 V$$$$H_{(a...
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(v) 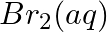 and
and 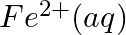
Solution: When it comes to electrochemistry, the term "standard electrode potential" refers to the value obtained by measuring the standard emf of a cell in which molecular hydrogen under standard...
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(iii) 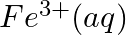 and
and 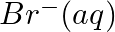
(iv) 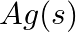 and
and 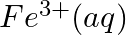
Solution: When it comes to electrochemistry, the term "standard electrode potential" refers to the value obtained by measuring the standard emf of a cell in which molecular hydrogen under standard...
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i) 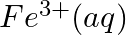 and
and 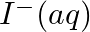
(ii) 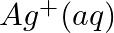 and
and 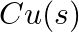
Solution: When it comes to electrochemistry, the term "standard electrode potential" refers to the value obtained by measuring the standard emf of a cell in which molecular hydrogen under standard...
Three electrolytic cells A, B,C containing solutions of 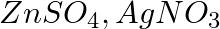 and
and 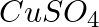 , respectively are connected in series. A steady current of
, respectively are connected in series. A steady current of 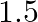 amperes was passed through them until
amperes was passed through them until 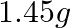 of silver was deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
of silver was deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
According to the information given in the question the reaction will be,$$A g_{(a q)}^{+}+e^{-} \rightarrow A g_{(s)}$$i.e., $108 g$ of $Ag$ is deposited by $96487 C$.Therefore, $1.45 g$ of $Ag$ is...
A solution of 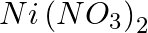 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Solution: As a result of the reaction, it is $$N i^{2+}+2 e^{-} \rightarrow N i_{(s)}+e^{-}$$ Nickel deposited by $2 \times 96487 C =58.71 g$ Therefore, nickel deposited by $6000 C =\frac{58.71...
How much electricity is required in coulomb for the oxidation of
(i) 1 mol of 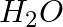 to
to 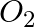 ?
?
(ii) 1 mol of FeO to 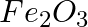 ?
?
Solution: (i) From given data, we write the equations that can be derived$$H_{2} O \rightarrow H_{2}+\frac{1}{2} O_{2} $$ We can say that : $$ O^{2-} \rightarrow \frac{1}{2} O_{2}+2...
How much electricity in terms of Faraday is required to produce
(i) 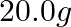 of
of 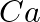 from molten
from molten 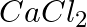 ?
?
(ii) 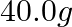 of
of 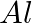 from molten
from molten 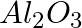 ?
?
Solution: (i) From given data, we write the equation of cell, $$Ca ^{2+}+2 e^{-} \rightarrow Ca$$ Evaluating the value of electricity in terms of Faraday we have, Electricity required to produce $40...
How much charge is required for the following reductions:
(i) 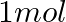 of
of 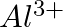 to
to 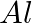 ?
?
(ii) 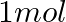 of
of 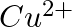 to
to 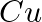 ?
?
(iii) 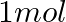 of
of 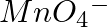 to
to 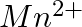 ?
?
Solution: As we know that, 1 mole of electron has a charge of 1 faraday (i) $A l^{3+}+3 e^{-} \rightarrow A l$Required charge $=3 F$$=3 \times 96487 C$$=289461 C$(ii) $C u^{2+}+2 e^{-} \rightarrow C...
Conductivity of 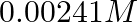 acetic acid is
acetic acid is 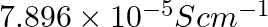 . Calculate its molar conductivity. If
. Calculate its molar conductivity. If 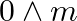 for acetic acid is
for acetic acid is 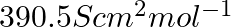 , what is its dissociation constant?
, what is its dissociation constant?
Solution: Given, $K =7.896 \times 10^{-5} S m ^{-1} c$ $$=0.00241 mol L ^{-1}$$As we know, molar conductivity, $\Lambda_{m}=\frac{k}{c}$ Substituting the value of known parameters we...
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below. Calculate Λm for all concentrations and draw a plot between Λm and c½. Find the value of 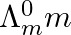
Solution: Evaluating the value of Λm for each case, Given,$$K =1.237 \times 10^{-2} S m -1, c =0.001 M$$Then, $K =1.237 \times 10^{-4} S cm ^{-1}, c ^{1 / 2}=0.0316 M ^{1 /...
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if the conductivity of 0.001M KCl solution at 298 K is 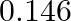

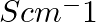
Solution: Given,Conductivity, $k =0.146 \times 10^{-3} S cm -1$Resistance, $R =1500 \Omega$Concept : We know the formula is, $$ \text { Cell constant }= k \times R$$ Calculation: Substuting the...
The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 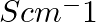 . Calculate its molar conductivity.
. Calculate its molar conductivity.
Solution: Given, $K =0.0248 S cm ^{-1} C$$$c =0.20 M$$ Concept: We know that Molar conductivity, $\Lambda_{m}=\frac{k \times 1000}{c}$ Calculation: Substituting the value of known parameter,...
Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Solution: Conductivity: The conductivity of a solution is defined as the conductance of a solution with a length of 1 cm and an area of cross-section of 1 sq. cm and a cross-sectional area of 1 sq....
In the button cells widely used in watches and other devices, the following reaction takes place. Determine ∆rGJ and EJ for the reaction.
Solution: Given, $E_0 = 1.104 V$ We know that, $$\Delta_{r} G^{\Theta}=-n F E^{\Theta}$$ Thus substituting the value of parmeters. = −2 × 96487 × 1.04 = −213043.296 J = −213.04...
Write the Nernst equation and emf of the following cells at 298 K: (i) 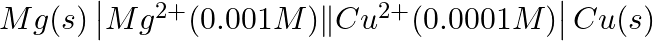
(ii) 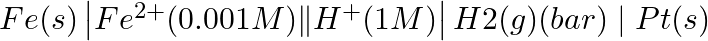
Solution: Nernst equation in electrochemistry is a relationship between the reduction potential of a reaction (either a half-cell or full-cell reaction) and various parameters such as the standard...
Write the Nernst equation and emf of the following cells at 298 K: (iii) 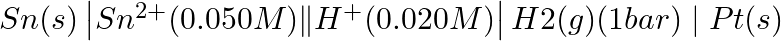
(iv) 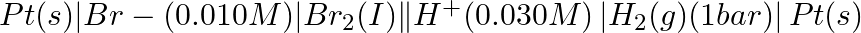
Solution: Nernst equation in electrochemistry is a relationship between the reduction potential of a reaction (either a half-cell or full-cell reaction) and various parameters such as the standard...
Calculate the standard cell potentials of galvanic cell in which the following reactions take place: (i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd (ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s) Calculate the ∆rGJ and equilibrium constant of the reactions.
Solution: (i) Given: $E_{C r^{3+} / C r}^{\Theta}=0.74 V$ $$E_{C d^{2+} / C d}^{\Theta}=-0.40 V$$The galvanic cell of the given reaction is represented as :$$C r_{(s)}\left|C r_{(a q)}^{3+} | C d_{a...
Depict the galvanic cell in which the reaction 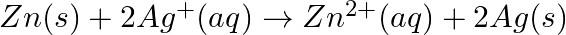 takes place. Further show: (i) Which of the electrode is negatively charged? (ii) The carriers of the current in the cell. (iii) Individual reaction at each electrode.
takes place. Further show: (i) Which of the electrode is negatively charged? (ii) The carriers of the current in the cell. (iii) Individual reaction at each electrode.
Solution: It is an electrochemical cell named after the inventors Luigi Galvani and Alessandro Volta, respectively, in which an electric current is generated through spontaneous reactions. The...
Given the standard electrode potentials,
K+/K = –2.93V
Ag+/Ag = 0.80V,
Hg2+/Hg = 0.79V
Mg2+/Mg = –2.37 V,
Cr3+/Cr = – 0.74V
Solution: As the reduction potential is reduced, the reducing power increases proportionally. The following are the standard electrode potentials in ascending order (increasing order): K+/K <...
Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg, and Zn
Solution: According to their reactivity, the following metals replaces in the following order: magnesium, aluminum, zinc, iron, and copper. Magnesium > Aluminum > Zinc > Copper
