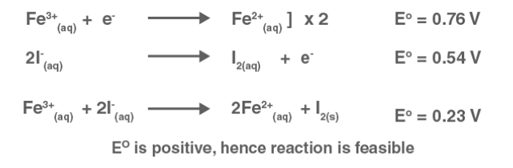
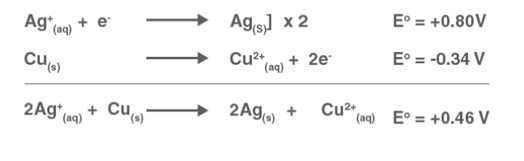
Solution:
When it comes to electrochemistry, the term “standard electrode potential” refers to the value obtained by measuring the standard emf of a cell in which molecular hydrogen under standard pressure is oxidized to solvated protons by connecting the left-hand electrode.