Consider the reactions (A) H2O2 + 2HI → I2 + 2H2O (B) HOCl + H2O2 → H3O++ Cl–+ O2 Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen peroxide is ________. (i) an oxidising agent in both (A) and (B) (ii) an oxidising agent in (A) and reducing agent in (B) (iii) a reducing agent in (A) and oxidising agent in (B) (iv) a reducing agent in both (A) and (B)
Solution:
Option (ii) is the answer.
(A) 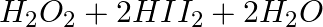
Iodine undergoes oxidation, transitioning from the -1 oxidation state to the 0 oxidation state. As a result, H2O2 works as an oxidising agent and undergoes a reduction process.
(B) 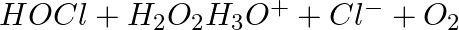
The oxidation state of chlorine is changed from +1 to -1 during the reduction process. As a result, H2O2 serves as a reducing agent while also becoming oxidised.
![]()
![]()
