Solution:
Hex-2-ene is addressed as displayed beneath:
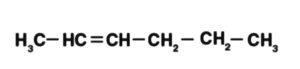
Mathematical isomers of hex-2-ene are as per the following:
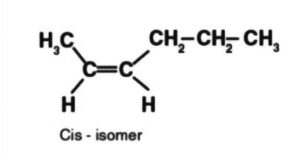
A cis intensifies dipole second is equivalent to the amount of the C – CH3 bond’s dipole minutes, and the C – CH2CH3 bonds that both demonstration a similar way.
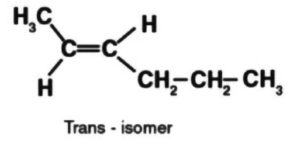
Then again, a trans-compound dipole second is the aftereffect of the dipole snapshots of C – CH3 bonds and C – CH2CH3 bonds both acting in inverse ways
As cis-isomer is polar than trans-isomer. Along these lines, the higher the extremity, the more prominent the intermolecular dipole-dipole connection and the requirement for more hotness to break the bonds. The bubbling will be higher, thusly.
Thusly, cis-isomer of a compound will have a higher edge of boiling over than trans-isomer of that compound.
