Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid.
Sulphuric acid helps in the formation of ethane as it is a dehydrating agent and also is a catalyst.
Reaction Involved:
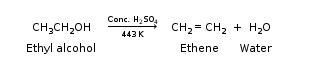
Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid.
Sulphuric acid helps in the formation of ethane as it is a dehydrating agent and also is a catalyst.
Reaction Involved:
