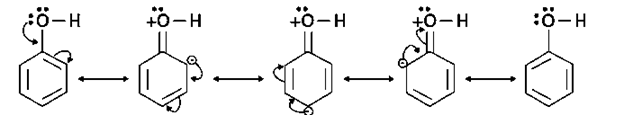
Because the – OH group works as an electron-donating group, the electron density in the benzene ring increases. The resonance structure of phenol, as seen below, clearly demonstrates this.
As a result, the benzene ring is activated towards electrophilic substitution.